Therapeutic role of histone deacetylase inhibition in an in vitro model of Graves' orbitopathy
- PMID: 39329199
- PMCID: PMC11465418
- DOI: 10.3892/mmr.2024.13342
Therapeutic role of histone deacetylase inhibition in an in vitro model of Graves' orbitopathy
Abstract
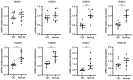
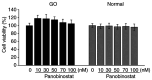
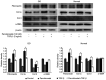
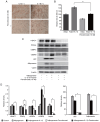
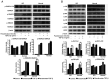
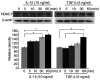
Graves' orbitopathy (GO), a manifestation of Graves' disease, is characterized by orbital fibroblast‑induced inflammation, leading to fibrosis or adipogenesis. Histone deacetylase (HDAC) serves a central role in autoimmune diseases and fibrosis. The present study investigated HDAC inhibition in orbital fibroblasts from patients with GO to evaluate its potential as a therapeutic agent. Primary cultured orbital fibroblasts were treated with an HDAC inhibitor, panobinostat, under the stimulation of IL‑1β, TGF‑β or adipogenic medium. Inflammatory cytokines, and fibrosis‑ and adipogenesis‑related proteins were analyzed using western blotting. The effects of panobinostat on HDAC mRNA expression were measured in GO orbital fibroblasts, and specific HDACs were inhibited using small interfering RNA transfection. Panobinostat significantly reduced the IL‑1β‑induced production of inflammatory cytokines and TGF‑β‑induced production of fibrosis‑related proteins. It also suppressed adipocyte differentiation and adipogenic transcription factor production. Furthermore, it significantly attenuated HDAC7 mRNA expression in GO orbital fibroblasts. In addition, the silencing of HDAC7 led to anti‑inflammatory and anti‑fibrotic effects. In conclusion, by inhibiting HDAC7 gene expression, panobinostat may suppress the production of inflammatory cytokines, profibrotic proteins and adipogenesis in GO orbital fibroblasts. The present in vitro study suggested that HDAC7 could be a potential therapeutic target for inhibiting the inflammatory, adipogenic and fibrotic mechanisms of GO.
Keywords: Graves' orbitopathy; histone deacetylase 7; histone deacetylase inhibitor; orbital fibroblast; panobinostat.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Histone Deacetylase 4 Controls Extracellular Matrix Production in Orbital Fibroblasts from Graves' Ophthalmopathy Patients.Thyroid. 2021 Oct;31(10):1566-1576. doi: 10.1089/thy.2020.0948. Epub 2021 Aug 18. Thyroid. 2021. PMID: 34235979
-
Compound C exerts a therapeutic effect on Graves' orbitopathy via AMPK‑independent pathways.Int J Mol Med. 2025 May;55(5):83. doi: 10.3892/ijmm.2025.5524. Epub 2025 Mar 21. Int J Mol Med. 2025. PMID: 40116114
-
Therapeutic effect of nintedanib in orbital fibroblasts in patients with Graves' orbitopathy.Immunopharmacol Immunotoxicol. 2025 Jun;47(3):406-418. doi: 10.1080/08923973.2025.2491554. Epub 2025 Apr 27. Immunopharmacol Immunotoxicol. 2025. PMID: 40289264
-
Molecular biomarkers of Graves' ophthalmopathy.Exp Mol Pathol. 2019 Feb;106:1-6. doi: 10.1016/j.yexmp.2018.11.004. Epub 2018 Nov 8. Exp Mol Pathol. 2019. PMID: 30414981 Free PMC article. Review.
-
Current perspectives on the role of orbital fibroblasts in the pathogenesis of Graves' ophthalmopathy.Exp Eye Res. 2016 Jan;142:83-91. doi: 10.1016/j.exer.2015.02.007. Exp Eye Res. 2016. PMID: 26675405 Review.
References
-
- Rotondo Dottore G, Torregrossa L, Lanzolla G, Mariotti S, Menconi F, Piaggi P, Cristofani Mencacci L, Posarelli C, Maglionico MN, Dallan I, et al. Role of the mononuclear cell infiltrate in Graves' orbitopathy (GO): Results of a large cohort study. J Endocrinol Invest. 2022;45:563–572. doi: 10.1007/s40618-021-01692-4. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

