Overview of a national endoscopy database: The Trans.IT database and its impact on data registration quality
- PMID: 39329225
- PMCID: PMC11578836
- DOI: 10.1002/ueg2.12669
Overview of a national endoscopy database: The Trans.IT database and its impact on data registration quality
Abstract
Background: The Trans.IT database is a national gastrointestinal (GI) endoscopy database developed in 2012. It automatically collects anonymous data from GI endoscopy procedures in a centralized database. All endoscopists use a structured reporting tool for uniform data collection. In this study, we aim to provide an overview of the database and to evaluate its impact on data registration quality.
Methods: We used all ERCPs, colonoscopies and colorectal cancer (CRC)-screening colonoscopies performed between 2016 and 2020. We excluded centers joining after 2016 and patients below age 18. Data registration quality for ERCPs included completeness of data for: intention of ERCP, Schutz score, ASA classification, papillary status (virgin or previous sphincterotomy), cannulation (success or failure to cannulate the desired duct) and procedural success. For colonoscopies: indication, ASA-classification, Boston Bowel Preparation Score (BBPS), cecal intubation, polyp detection rate (PDR). For CRC-screening colonoscopies, ASA-classification, BBPS, cecal intubation, PDR and adenoma detection rate (ADR).
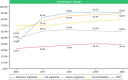
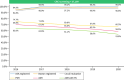
Results: A total of 14,156 ERCPs, 150,962 colonoscopies and 37,199 colorectal cancer screening colonoscopies were included in our analysis. For ERCPs, registration of procedural intention, Schutz score, ASA classification, papillary status, cannulation and procedural success improved from 34.9%, 32.7%, 72.6%, 36.5%, 34.6%, 27.2% in 2016, to 86.4%, 84.6%, 97.4%, 86.4%, 82.1%, 84.0%, respectively, in 2020. For non-screening colonoscopies, registration of indication, ASA classification, BBPS, cecal intubation and PDR improved from 40.4%, 60.5%, 47.6%, 69.8% and 32.3% in 2016 to 90.3%, 88.9%, 59.8%, 79.1% and 39.1%, respectively, in 2020. For CRC-cancer screening colonoscopy registration equaled outcome, PDR and ADR changed from 74.7% to 63.6% in 2016 to 66.3% and 53.8% in 2020, respectively.
Conclusions: The quality of endoscopy data registration has consistently improved over the years by using the Trans.IT database. This is most likely the result of feedback to performing endoscopists to review performance in real-time online and progressive awareness of quality of data registration.
Keywords: ASA classification; Boston bowel preparation score; CRC; ERCP; adenoma detection rate; colonoscopy; colorectal cancer; gastrointestinal; registry; screening.
© 2024 The Author(s). United European Gastroenterology Journal published by Wiley Periodicals LLC on behalf of United European Gastroenterology.
Conflict of interest statement
F. Theunissen: no conflicting interests. P. C. J. ter Borg: no conflicting interests. R. J. Ouwendijk: Research grants from Janssen Netherlands, Norgine and the Coolsingel Foundation. M. J. Bruno: Boston Scientific; Consultant, support for industry and investigator‐initiated studies; Cook Medical; Consultant, support for industry and investigator initiated studies; Pentax Medical; support for investigator initiated studies; Mylan; support for investigator initiated studies; ChiRoStim; support for investigator initiated studies. P. D. Siersema: Research grants from Norgine, Pentax, FujiFilm, Microtech, Sanofi, Magentiq Eye, for investigator‐initiated studies; Advisory Board of Sanofi.
Figures




References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

