Involvement of a battery of investigated genes in lipid droplet pathophysiology and associated comorbidities
- PMID: 39329369
- PMCID: PMC11445895
- DOI: 10.1080/21623945.2024.2403380
Involvement of a battery of investigated genes in lipid droplet pathophysiology and associated comorbidities
Abstract
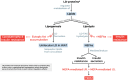
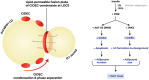
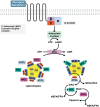
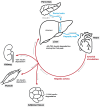
Lipid droplets (LDs) are highly specialized energy storage organelles involved in the maintenance of lipid homoeostasis by regulating lipid flux within white adipose tissue (WAT). The physiological function of adipocytes and LDs can be compromised by mutations in several genes, leading to NEFA-induced lipotoxicity, which ultimately manifests as metabolic complications, predominantly in the form of dyslipidemia, ectopic fat accumulation, and insulin resistance. In this review, we delineate the effects of mutations and deficiencies in genes - CIDEC, PPARG, BSCL2, AGPAT2, PLIN1, LIPE, LMNA, CAV1, CEACAM1, and INSR - involved in lipid droplet metabolism and their associated pathophysiological impairments, highlighting their roles in the development of lipodystrophies and metabolic dysfunction.
Keywords: Lipid droplet formation; adipogenesis; insulin resistance; lipid droplet hydrolysis; lipodystrophy.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
