The feasibility of establishing a hamster model for HBV infection: in vitro evidence
- PMID: 39329526
- PMCID: PMC11559161
- DOI: 10.1128/mbio.02615-24
The feasibility of establishing a hamster model for HBV infection: in vitro evidence
Abstract
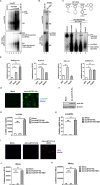
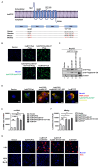
Chronic hepatitis B virus (HBV) infection remains a significant public health burden with no cure currently available. The research to cure HBV has long been hampered by the lack of immunocompetent small animal models capable of supporting HBV infection. Here, we set out to explore the feasibility of the golden Syrian hamster as an immunocompetent small rodent model for HBV infection. We first started with in vitro assessments of the HBV replication cycle in primary hamster hepatocytes (PHaHs) by adenoviral HBV (Ad-HBV) transduction. Our results demonstrated that PHaHs support HBV reverse transcription and subsequent cccDNA formation via the intracellular recycling pathway. Next, with luciferase reporter assays, we confirmed that PHaHs support the activities of all HBV major promoters. Then, we transduced PHaHs with an adenoviral vector expressing HBV receptor human Na+/taurocholate cotransporting polypeptide NTCP (Ad-huNTCP), followed by HBV inoculation. While the untransduced PHaHs did not support HBV infection, Ad-huNTCP-transduced PHaHs supported de novo cccDNA formation, viral mRNA transcription, and expression of viral antigens. We then humanized the amino acid (aa) residues of hamster NTCP (haNTCP) critical for HBV entry, aa84-87 and aa157-165, and transfected HepG2 cells with constructs expressing wild-type haNTCP and humanized-haNTCP, H84R/P87N and H84R/P87N/G157K/M160V/M165L, respectively, followed by HBV inoculation. The results showed that the humanization of H84R/P87N alone was sufficient to support HBV infection at a level comparable to that supported by huNTCP. Taken together, the above in vitro evidence supports the future direction of humanizing haNTCP for HBV infection in vivo.IMPORTANCEOne of the biggest challenges in developing an HBV cure is the lack of immunocompetent animal models susceptible to HBV infection. Developing such models in mice has been unsuccessful due to the absence of a functional HBV receptor, human NTCP (huNTCP), and the defect in supporting viral cccDNA formation. In search of alternative models, we report herein multiple lines of in vitro evidence for developing a golden Syrian hamster model for HBV infection. We demonstrate that the primary hamster hepatocytes (PHaHs) support HBV replication, transcription, and cccDNA formation, and PHaHs are susceptible to de novo HBV infection in the presence of huNTCP. Furthermore, expressing hamster NTCP with two humanized residues critical for HBV entry renders HepG2 cells permissive to HBV infection. Thus, our work lays a solid foundation for establishing a gene-edited hamster model that expresses humanized NTCP for HBV infection in vivo.
Keywords: NTCP; cccDNA; hamster hepatocytes; hepatitis B virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Robust Human and Murine Hepatocyte Culture Models of Hepatitis B Virus Infection and Replication.J Virol. 2018 Nov 12;92(23):e01255-18. doi: 10.1128/JVI.01255-18. Print 2018 Dec 1. J Virol. 2018. PMID: 30232184 Free PMC article.
-
Viral entry of hepatitis B and D viruses and bile salts transportation share common molecular determinants on sodium taurocholate cotransporting polypeptide.J Virol. 2014 Mar;88(6):3273-84. doi: 10.1128/JVI.03478-13. Epub 2014 Jan 3. J Virol. 2014. PMID: 24390325 Free PMC article.
-
Sodium taurocholate cotransporting polypeptide is the limiting host factor of hepatitis B virus infection in macaque and pig hepatocytes.Hepatology. 2017 Sep;66(3):703-716. doi: 10.1002/hep.29112. Epub 2017 Jul 18. Hepatology. 2017. PMID: 28195359
-
Innovative HBV Animal Models Based on the Entry Receptor NTCP.Viruses. 2020 Jul 30;12(8):828. doi: 10.3390/v12080828. Viruses. 2020. PMID: 32751581 Free PMC article. Review.
-
From DCPD to NTCP: the long journey towards identifying a functional hepatitis B virus receptor.Clin Mol Hepatol. 2015 Sep;21(3):193-9. doi: 10.3350/cmh.2015.21.3.193. Epub 2015 Sep 30. Clin Mol Hepatol. 2015. PMID: 26523264 Free PMC article. Review.
Cited by
-
Screening of different species reveals cat hepatocytes support HBV infection.PLoS Pathog. 2025 Aug 4;21(8):e1013390. doi: 10.1371/journal.ppat.1013390. eCollection 2025 Aug. PLoS Pathog. 2025. PMID: 40758741 Free PMC article.
-
Identification of NTCP animal orthologs supporting hepatitis B virus binding and infection.J Virol. 2025 Apr 15;99(4):e0183324. doi: 10.1128/jvi.01833-24. Epub 2025 Mar 5. J Virol. 2025. PMID: 40042307 Free PMC article.
-
Host 3' flap endonuclease Mus81 plays a critical role in trimming the terminal redundancy of hepatitis B virus relaxed circular DNA during covalently closed circular DNA formation.PLoS Pathog. 2025 Feb 6;21(2):e1012918. doi: 10.1371/journal.ppat.1012918. eCollection 2025 Feb. PLoS Pathog. 2025. PMID: 39913382 Free PMC article.
References
-
- Revill PA, Chisari FV, Block JM, Dandri M, Gehring AJ, Guo H, Hu J, Kramvis A, Lampertico P, Janssen HLA, Levrero M, Li W, Liang TJ, Lim S-G, Lu F, Penicaud MC, Tavis JE, Thimme R, Zoulim F, Members of the ICE-HBV Working Groups, ICE-HBV Stakeholders Group Chairs, ICE-HBV Senior Advisors . 2019. A global scientific strategy to cure hepatitis B. Lancet Gastroenterol Hepatol 4:545–558. doi:10.1016/S2468-1253(19)30119-0 - DOI - PMC - PubMed
-
- Yan H, Zhong G, Xu G, He W, Jing Z, Gao Z, Huang Y, Qi Y, Peng B, Wang H, Fu L, Song M, Chen P, Gao W, Ren B, Sun Y, Cai T, Feng X, Sui J, Li W. 2012. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 3:e00049. doi:10.7554/eLife.00049 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- R01 AI134818/AI/NIAID NIH HHS/United States
- R01 AI183883/AI/NIAID NIH HHS/United States
- R21 AI179929/AI/NIAID NIH HHS/United States
- P30 DK120531/DK/NIDDK NIH HHS/United States
- R01 AI150255/AI/NIAID NIH HHS/United States
- R01 AI110762/AI/NIAID NIH HHS/United States
- P30 CA047904/CA/NCI NIH HHS/United States
- R24 DK139775/DK/NIDDK NIH HHS/United States
- R01AI110762, R01AI150255/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01AI183883/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- P30CA047904/HHS | NIH | National Cancer Institute (NCI)
LinkOut - more resources
Full Text Sources
