mRNA localization and thylakoid protein biogenesis in the filamentous heterocyst-forming cyanobacterium Anabaena sp. PCC 7120
- PMID: 39329528
- PMCID: PMC11500504
- DOI: 10.1128/jb.00328-24
mRNA localization and thylakoid protein biogenesis in the filamentous heterocyst-forming cyanobacterium Anabaena sp. PCC 7120
Abstract
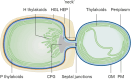
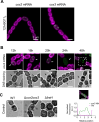
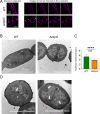
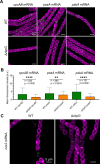
Heterocyst-forming cyanobacteria such as Anabaena (Nostoc) sp. PCC 7120 exhibit extensive remodeling of their thylakoid membranes during heterocyst differentiation. Here we investigate the sites of translation of thylakoid membrane proteins in Anabaena vegetative cells and developing heterocysts, using mRNA fluorescent in situ hybridization (FISH) to detect the location of specific mRNA species. We probed mRNAs encoding reaction center core components and the heterocyst-specific terminal oxidases Cox2 and Cox3. As in unicellular cyanobacteria, the mRNAs encoding membrane-integral thylakoid proteins are concentrated in patches at the inner face of the thylakoid membrane system, adjacent to the central cytoplasm. These patches mark the putative sites of translation and membrane insertion of these proteins. Oxidase activity in mature heterocysts is concentrated in the specialized "honeycomb" regions of the thylakoid membranes close to the cell poles. However, cox2 and cox3 mRNAs remain evenly distributed over the inner face of the thylakoids, implying that oxidase proteins migrate extensively after translation to reach their destination in the honeycomb membranes. The RNA-binding protein RbpG is the closest Anabaena homolog of Rbp3 in the unicellular cyanobacterium Synechocystis sp. PCC 6803, which we previously showed to be crucial for the correct location of photosynthetic mRNAs. An rbpG null mutant shows decreased cellular levels of photosynthetic mRNAs and photosynthetic complexes, coupled with perturbations to thylakoid membrane organization and lower efficiency of the Photosystem II repair cycle. This suggests that the chaperoning of photosynthetic mRNAs by RbpG is important for the correct coordination of thylakoid protein translation and assembly.IMPORTANCECyanobacteria have a complex thylakoid membrane system which is the site of the photosynthetic light reactions as well as most of the respiratory activity in the cell. Protein targeting to the thylakoids and the spatial organization of thylakoid protein biogenesis remain poorly understood. Further complexity is found in some filamentous cyanobacteria that produce heterocysts, specialized nitrogen-fixing cells in which the thylakoid membranes undergo extensive remodeling. Here we probe mRNA locations to reveal thylakoid translation sites in a heterocyst-forming cyanobacterium. We identify an RNA-binding protein important for the correct co-ordination of thylakoid protein translation and assembly, and we demonstrate the effectiveness of mRNA fluorescent in situ hybridization (FISH) as a way to probe cell-specific gene expression in multicellular cyanobacteria.
Keywords: RNA binding protein; cyanobacteria; heterocyst; mRNA; membrane proteins; thylakoid membrane.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
ThyD Is a Thylakoid Membrane Protein Influencing Cell Division and Acclimation to High Light in the Multicellular Cyanobacterium Anabaena sp. Strain PCC 7120.Mol Microbiol. 2025 Jan;123(1):31-47. doi: 10.1111/mmi.15335. Epub 2024 Dec 4. Mol Microbiol. 2025. PMID: 39630597 Free PMC article.
-
A TonB-Like Protein, SjdR, Is Involved in the Structural Definition of the Intercellular Septa in the Heterocyst-Forming Cyanobacterium Anabaena.mBio. 2021 Jun 29;12(3):e0048321. doi: 10.1128/mBio.00483-21. Epub 2021 Jun 8. mBio. 2021. PMID: 34101487 Free PMC article.
-
Locations of membrane protein production in a cyanobacterium.J Bacteriol. 2023 Oct 26;205(10):e0020923. doi: 10.1128/jb.00209-23. Epub 2023 Oct 3. J Bacteriol. 2023. PMID: 37787518 Free PMC article.
-
Thylakoid membrane function in heterocysts.Biochim Biophys Acta. 2016 Mar;1857(3):309-19. doi: 10.1016/j.bbabio.2015.10.016. Epub 2015 Nov 9. Biochim Biophys Acta. 2016. PMID: 26545609 Review.
-
ATP-binding cassette transporters of the multicellular cyanobacterium Anabaena sp. PCC 7120: a wide variety for a complex lifestyle.FEMS Microbiol Lett. 2018 Feb 1;365(4). doi: 10.1093/femsle/fny012. FEMS Microbiol Lett. 2018. PMID: 29360977 Review.
Cited by
-
ThyD Is a Thylakoid Membrane Protein Influencing Cell Division and Acclimation to High Light in the Multicellular Cyanobacterium Anabaena sp. Strain PCC 7120.Mol Microbiol. 2025 Jan;123(1):31-47. doi: 10.1111/mmi.15335. Epub 2024 Dec 4. Mol Microbiol. 2025. PMID: 39630597 Free PMC article.
-
The RRM domain-containing protein Rbp3 interacts with ribosomes and the 3' ends of mRNAs encoding photosynthesis proteins.Proc Natl Acad Sci U S A. 2025 Jul;122(26):e2506275122. doi: 10.1073/pnas.2506275122. Epub 2025 Jun 24. Proc Natl Acad Sci U S A. 2025. PMID: 40553498
-
Spatial proteomics reveals signal sequence characteristics correlated with localization in cyanobacteria.Plant Physiol. 2025 Aug 4;198(4):kiaf186. doi: 10.1093/plphys/kiaf186. Plant Physiol. 2025. PMID: 40794799 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

