Nanoparticles as Drug Delivery Vehicles for People with Cystic Fibrosis
- PMID: 39329596
- PMCID: PMC11430251
- DOI: 10.3390/biomimetics9090574
Nanoparticles as Drug Delivery Vehicles for People with Cystic Fibrosis
Abstract
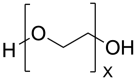
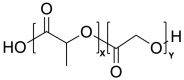
Cystic Fibrosis (CF) is a life-shortening, genetic disease that affects approximately 145,000 people worldwide. CF causes a dehydrated mucus layer in the lungs, leading to damaging infection and inflammation that eventually result in death. Nanoparticles (NPs), drug delivery vehicles intended for inhalation, have become a recent source of interest for treating CF and CF-related conditions, and many formulations have been created thus far. This paper is intended to provide an overview of CF and the effect it has on the lungs, the barriers in using NP drug delivery vehicles for treatment, and three common material class choices for these NP formulations: metals, polymers, and lipids. The materials to be discussed include gold, silver, and iron oxide metallic NPs; polyethylene glycol, chitosan, poly lactic-co-glycolic acid, and alginate polymeric NPs; and lipid-based NPs. The novelty of this review comes from a less specific focus on nanoparticle examples, with the focus instead being on the general theory behind material function, why or how a material might be used, and how it may be preferable to other materials used in treating CF. Finally, this paper ends with a short discussion of the two FDA-approved NPs for treatment of CF-related conditions and a recommendation for the future usage of NPs in people with Cystic Fibrosis (pwCF).
Keywords: FDA; antibiotics; cystic fibrosis; drug delivery; lipids; metals; nanoparticles; polymers; treatments.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Antimicrobial efficacy of tobramycin polymeric nanoparticles for Pseudomonas aeruginosa infections in cystic fibrosis: formulation, characterisation and functionalisation with dornase alfa (DNase).J Control Release. 2015 Jan 28;198:55-61. doi: 10.1016/j.jconrel.2014.11.022. Epub 2014 Dec 4. J Control Release. 2015. PMID: 25481442
-
Hybrid Lipid/Polymer Nanoparticles to Tackle the Cystic Fibrosis Mucus Barrier in siRNA Delivery to the Lungs: Does PEGylation Make the Difference?ACS Appl Mater Interfaces. 2022 Feb 16;14(6):7565-7578. doi: 10.1021/acsami.1c14975. Epub 2022 Feb 2. ACS Appl Mater Interfaces. 2022. PMID: 35107987 Free PMC article.
-
Exercise as an Airway Clearance Technique in people with Cystic Fibrosis (ExACT-CF): rationale and study protocol for a randomised pilot trial.NIHR Open Res. 2022 Dec 19;2:64. doi: 10.3310/nihropenres.13347.1. eCollection 2022. NIHR Open Res. 2022. PMID: 37881306 Free PMC article.
-
Theranostic Nanoparticles for RNA-Based Cancer Treatment.Acc Chem Res. 2019 Jun 18;52(6):1496-1506. doi: 10.1021/acs.accounts.9b00101. Epub 2019 May 28. Acc Chem Res. 2019. PMID: 31135134 Free PMC article. Review.
-
Nanoparticle-based platforms for targeted drug delivery to the pulmonary system as therapeutics to curb cystic fibrosis: A review.J Microbiol Methods. 2024 Feb-Mar;217-218:106876. doi: 10.1016/j.mimet.2023.106876. Epub 2023 Dec 20. J Microbiol Methods. 2024. PMID: 38135160 Review.
References
-
- About Cystic Fibrosis | Cystic Fibrosis Foundation. [(accessed on 21 April 2024)]. Available online: https://www.cff.org/intro-cf/about-cystic-fibrosis.
-
- Understanding Changes in Life Expectancy | Cystic Fibrosis Foundation. [(accessed on 21 April 2024)]. Available online: https://www.cff.org/managing-cf/understanding-changes-life-expectancy.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

