Encapsulation of Inositol Hexakisphosphate with Chitosan via Gelation to Facilitate Cellular Delivery and Programmed Cell Death in Human Breast Cancer Cells
- PMID: 39329673
- PMCID: PMC11429465
- DOI: 10.3390/bioengineering11090931
Encapsulation of Inositol Hexakisphosphate with Chitosan via Gelation to Facilitate Cellular Delivery and Programmed Cell Death in Human Breast Cancer Cells
Abstract
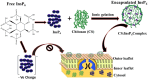
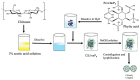
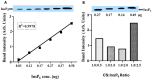
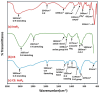
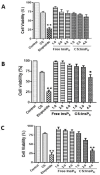
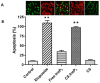
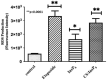
Inositol hexakisphosphate (InsP6) is the most abundant inositol polyphosphate both in plant and animal cells. Exogenous InsP6 is known to inhibit cell proliferation and induce apoptosis in cancerous cells. However, cellular entry of exogenous InsP6 is hindered due to the presence of highly negative charge on this molecule. Therefore, to enhance the cellular delivery of InsP6 in cancerous cells, InsP6 was encapsulated by chitosan (CS), a natural polysaccharide, via the ionic gelation method. Our hypothesis is that encapsulated InsP6 will enter the cell more efficiently to trigger its apoptotic effects. The incorporation of InsP6 into CS was optimized by varying the ratios of the two and confirmed by InsP6 analysis via polyacrylamide gel electrophoresis (PAGE) and atomic absorption spectrophotometry (AAS). The complex was further characterized by Scanning Electron Microscopy (SEM) and Fourier Transform Infrared Spectroscopy (FTIR) for physicochemical changes. The data indicated morphological changes and changes in the spectral properties of the complex upon encapsulation. The encapsulated InsP6 enters human breast cancer MCF-7 cells more efficiently than free InsP6 and triggers apoptosis via a mechanism involving the production of reactive oxygen species (ROS). This work has potential for developing cancer therapeutic applications utilizing natural compounds that are likely to overcome the severe toxic effects associated with synthetic chemotherapeutic drugs.
Keywords: apoptosis; breast cancer; chitosan; cytotoxicity; encapsulation; inositol polyphosphates; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Role of inositol polyphosphates in programed cell death in Dictyostelium discoideum and its developmental life cycle.Mol Cell Biochem. 2018 Dec;449(1-2):237-250. doi: 10.1007/s11010-018-3360-6. Epub 2018 Apr 20. Mol Cell Biochem. 2018. PMID: 29679279
-
Accumulation of [3H]-inositol into inositol polyphosphates during development of Dictyostelium.Biochem Biophys Res Commun. 1991 Nov 27;181(1):191-6. doi: 10.1016/s0006-291x(05)81400-7. Biochem Biophys Res Commun. 1991. PMID: 1958187
-
The intracellular distribution of inositol polyphosphates in HL60 promyeloid cells.Biochem J. 1994 Oct 15;303 ( Pt 2)(Pt 2):517-25. doi: 10.1042/bj3030517. Biochem J. 1994. PMID: 7980412 Free PMC article.
-
New options of cancer treatment employing InsP6.Biochem Pharmacol. 2019 May;163:206-214. doi: 10.1016/j.bcp.2019.02.024. Epub 2019 Feb 21. Biochem Pharmacol. 2019. PMID: 30797871 Review.
-
Inositol phosphates have novel anticancer function.J Nutr. 1995 Mar;125(3 Suppl):725S-732S. doi: 10.1093/jn/125.3_Suppl.725S. J Nutr. 1995. PMID: 7884558 Review.
Cited by
-
Targeted Delivery of Rapamycin via Epidermal Growth Factor Receptors in Pancreatic Cancer Cells Inhibits Cell Proliferation and Induces Apoptosis.ACS Omega. 2025 Jul 19;10(29):31762-31775. doi: 10.1021/acsomega.5c02820. eCollection 2025 Jul 29. ACS Omega. 2025. PMID: 40757331 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

