A 3D Epithelial-Mesenchymal Co-Culture Model of the Airway Wall Using Native Lung Extracellular Matrix
- PMID: 39329688
- PMCID: PMC11428669
- DOI: 10.3390/bioengineering11090946
A 3D Epithelial-Mesenchymal Co-Culture Model of the Airway Wall Using Native Lung Extracellular Matrix
Abstract
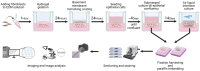
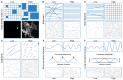
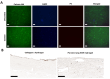
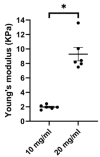
Chronic obstructive pulmonary disease (COPD) is a chronic lung disease characterized by ongoing inflammation, impaired tissue repair, and aberrant interplay between airway epithelium and fibroblasts, resulting in an altered extracellular matrix (ECM) composition. The ECM is the three-dimensional (3D) scaffold that provides mechanical support and biochemical signals to cells, now recognized not only as a consequence but as a potential driver of disease progression. To elucidate how the ECM influences pathophysiological changes occurring in COPD, in vitro models are needed that incorporate the ECM. ECM hydrogels are a novel experimental tool for incorporating the ECM in experimental setups. We developed an airway wall model by combining lung-derived ECM hydrogels with a co-culture of primary human fibroblasts and epithelial cells at an air-liquid interface. Collagen IV and a mixture of collagen I, fibronectin, and bovine serum albumin were used as basement membrane-mimicking coatings. The model was initially assembled using porcine lung-derived ECM hydrogels and subsequently with COPD and non-COPD human lung-derived ECM hydrogels. The resulting 3D construct exhibited considerable contraction and supported co-culture, resulting in a differentiated epithelial layer. This multi-component 3D model allows the investigation of remodelling mechanisms, exploring ECM involvement in cellular crosstalk, and holds promise as a model for drug discovery studies exploring ECM involvement in cellular interactions.
Keywords: COPD; TWOMBLI; extracellular matrix.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the ma script, or in the decision to publish the results.
Figures

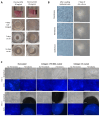
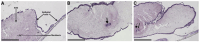




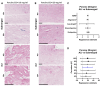

Similar articles
-
3D in vitro hydrogel models to study the human lung extracellular matrix and fibroblast function.Respir Res. 2023 Oct 5;24(1):242. doi: 10.1186/s12931-023-02548-6. Respir Res. 2023. PMID: 37798767 Free PMC article. Review.
-
Three dimensional fibrotic extracellular matrix directs microenvironment fiber remodeling by fibroblasts.Acta Biomater. 2024 Mar 15;177:118-131. doi: 10.1016/j.actbio.2024.02.008. Epub 2024 Feb 11. Acta Biomater. 2024. PMID: 38350556
-
Interleukin-1α drives the dysfunctional cross-talk of the airway epithelium and lung fibroblasts in COPD.Eur Respir J. 2016 Aug;48(2):359-69. doi: 10.1183/13993003.01911-2015. Epub 2016 Jul 13. Eur Respir J. 2016. PMID: 27418555
-
An in vitro model of fibrosis using crosslinked native extracellular matrix-derived hydrogels to modulate biomechanics without changing composition.Acta Biomater. 2022 Jul 15;147:50-62. doi: 10.1016/j.actbio.2022.05.031. Epub 2022 May 21. Acta Biomater. 2022. PMID: 35605955
-
What Have In Vitro Co-Culture Models Taught Us about the Contribution of Epithelial-Mesenchymal Interactions to Airway Inflammation and Remodeling in Asthma?Cells. 2020 Jul 15;9(7):1694. doi: 10.3390/cells9071694. Cells. 2020. PMID: 32679790 Free PMC article. Review.
References
-
- Eisner M.D., Anthonisen N., Coultas D., Kuenzli N., Perez-Padilla R., Postma D., Romieu I., Silverman E.K., Balmes J.R. An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010;182:693–718. doi: 10.1164/rccm.200811-1757ST. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

