The Oncoprotein Fra-2 Drives the Activation of Human Endogenous Retrovirus Env Expression in Adult T-Cell Leukemia/Lymphoma (ATLL) Patients
- PMID: 39329701
- PMCID: PMC11430398
- DOI: 10.3390/cells13181517
The Oncoprotein Fra-2 Drives the Activation of Human Endogenous Retrovirus Env Expression in Adult T-Cell Leukemia/Lymphoma (ATLL) Patients
Abstract
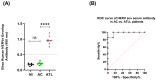
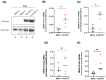
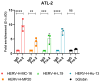
Human endogenous retroviruses (HERVs) are retroviral sequences integrated into 8% of the human genome resulting from ancient exogenous retroviral infections. Unlike endogenous retroviruses of other mammalian species, HERVs are mostly replication and retro-transposition defective, and their transcription is strictly regulated by epigenetic mechanisms in normal cells. A significant addition to the growing body of research reveals that HERVs' aberrant activation is often associated with offsetting diseases like autoimmunity, neurodegenerative diseases, cancers, and chemoresistance. Adult T-cell leukemia/lymphoma (ATLL) is a very aggressive and chemoresistant leukemia caused by the human T-cell leukemia virus type 1 (HTLV-1). The prognosis of ATLL remains poor despite several new agents being approved in the last few years. In the present study, we compare the expression of HERV genes in CD8+-depleted PBMCs from HTLV-1 asymptomatic carriers and patients with acute ATLL. Herein, we show that HERVs are highly upregulated in acute ATLL. Our results further demonstrate that the oncoprotein Fra-2 binds the LTR region and activates the transcription of several HERV families, including HERV-H and HERV-K families. This raises the exciting possibility that upregulated HERV expression could be a key factor in ATLL development and the observed chemoresistance, potentially leading to new therapeutic strategies and significantly impacting the field of oncology and virology.
Keywords: AP-1; ATLL; Fra-2; HERVs; leukemia.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Tobinai K. Current management of adult T-cell leukemia/lymphoma. Oncology. 2009;23:1250–1256. - PubMed
-
- Ikeda K., Oka M., Yamada Y., Soda H., Fukuda M., Kinoshita A., Tsukamoto K., Noguchi Y., Isomoto H., Kohno S., et al. Adult T-cell leukemia cells over-express the multidrug-resistance-protein (MRP) and lung-resistance-protein (LRP) genes. Int. J. Cancer. 1999;82:599–604. doi: 10.1002/(SICI)1097-0215(19990812)82:4<599::AID-IJC21>3.0.CO;2-R. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

