Structural and Functional Characterization of the Most Frequent Pathogenic PRKN Substitution p.R275W
- PMID: 39329724
- PMCID: PMC11430725
- DOI: 10.3390/cells13181540
Structural and Functional Characterization of the Most Frequent Pathogenic PRKN Substitution p.R275W
Abstract
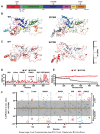
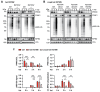
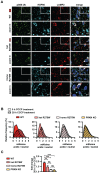
Mutations in the PINK1 and PRKN genes are the most frequent genetic cause of early-onset Parkinson disease. The pathogenic p.R275W substitution in PRKN is the most frequent substitution observed in patients, and thus far has been characterized mostly through overexpression models that suggest a possible gain of toxic misfunction. However, its effects under endogenous conditions are largely unknown. We used patient fibroblasts, isogenic neurons, and post-mortem human brain samples from carriers with and without PRKN p.R275W to assess functional impact. Immunoblot analysis and immunofluorescence were used to study mitophagy activation, and mitophagy execution was analyzed by flow cytometry of the reporter mitoKeima. The functional analysis was accompanied by structural investigation of PRKN p.R275W. We observed lower PRKN protein in fibroblasts with compound heterozygous p.R275W mutations. Isogenic neurons showed an allele-dose dependent decrease in PRKN protein. Lower PRKN protein levels were accompanied by diminished phosphorylated ubiquitin and decreased MFN2 modification. Mitochondrial degradation was also allele-dose dependently impaired. Consistently, PRKN protein levels were drastically reduced in human brain samples from p.R275W carriers. Finally, structural simulations showed significant changes in the closed form of PRKN p.R275W. Our data suggest that under endogenous conditions the p.R275W mutation results in a loss-of-function by destabilizing PRKN.
Keywords: PINK1; PRKN; Parkinson disease; mitophagy; parkin; ubiquitin.
Conflict of interest statement
Mayo Clinic, F.C.F. and W.S. hold a patent related to PRKN activators (Small Molecule Activators of Parkin Enzyme Function, US patent,11401255B2; 2 August 2022). D.P.N. receives research funding from Spark Therapeutics. All other authors declare they have no competing interests. Z.K.W. serves as PI or Co-PI on Biohaven Pharmaceuticals, Inc. (BHV4157-206), Vigil Neuroscience, Inc. (VGL101-01.002, VGL101-01.201, PET tracer development protocol, Csf1r biomarker and repository project, and ultra-high field MRI in the diagnosis and management of CSF1R-related adult-onset leukoencephalopathy with axonal spheroids and pigmented glia), and ONO-2808-03 projects/grants. He serves as Co-PI of the Mayo Clinic APDA Center for Advanced Research and as an external advisory board member for the Vigil Neuroscience, Inc., and as a consultant for Eli Lilly & Company and for NovoGlia, Inc. This research was conducted in compliance with Mayo Clinic conflict of interest policies.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

