A Novel Non-Invasive Murine Model of Neonatal Hypoxic-Ischemic Encephalopathy Demonstrates Developmental Delay and Motor Deficits with Activation of Inflammatory Pathways in Monocytes
- PMID: 39329733
- PMCID: PMC11429599
- DOI: 10.3390/cells13181551
A Novel Non-Invasive Murine Model of Neonatal Hypoxic-Ischemic Encephalopathy Demonstrates Developmental Delay and Motor Deficits with Activation of Inflammatory Pathways in Monocytes
Abstract
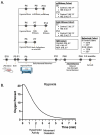
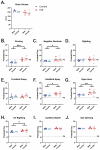
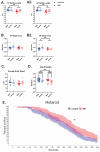
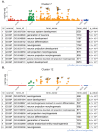
Neonatal hypoxic-ischemic encephalopathy (HIE) occurs in 1.5 per 1000 live births, leaving affected children with long-term motor and cognitive deficits. Few animal models of HIE incorporate maternal immune activation (MIA) despite the significant risk MIA poses to HIE incidence and diagnosis. Our non-invasive model of HIE pairs late gestation MIA with postnatal hypoxia. HIE pups exhibited a trend toward smaller overall brain size and delays in the ontogeny of several developmental milestones. In adulthood, HIE animals had reduced strength and gait deficits, but no difference in speed. Surprisingly, HIE animals performed better on the rotarod, an assessment of motor coordination. There was significant upregulation of inflammatory genes in microglia 24 h after hypoxia. Single-cell RNA sequencing (scRNAseq) revealed two microglia subclusters of interest following HIE. Pseudobulk analysis revealed increased microglia motility gene expression and upregulation of epigenetic machinery and neurodevelopmental genes in macrophages following HIE. No sex differences were found in any measures. These results support a two-hit noninvasive model pairing MIA and hypoxia as a model for HIE in humans. This model results in a milder phenotype compared to established HIE models; however, HIE is a clinically heterogeneous injury resulting in a variety of outcomes in humans. The pathways identified in our model of HIE may reveal novel targets for therapy for neonates with HIE.
Keywords: development; hypoxic-ischemic encephalopathy; macrophages; maternal immune activation; microglia; motor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Namusoke H., Nannyonga M.M., Ssebunya R., Nakibuuka V.K., Mworozi E. Incidence and short term outcomes of neonates with hypoxic ischemic encephalopathy in a Peri Urban teaching hospital, Uganda: A prospective cohort study. Matern. Health Neonatol. Perinatol. 2018;4:6. doi: 10.1186/s40748-018-0074-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

