Comparative Analysis of Inhibitory and Activating Immune Checkpoints PD-1, PD-L1, CD28, and CD86 in Non-Melanoma Skin Cancer
- PMID: 39329753
- PMCID: PMC11430031
- DOI: 10.3390/cells13181569
Comparative Analysis of Inhibitory and Activating Immune Checkpoints PD-1, PD-L1, CD28, and CD86 in Non-Melanoma Skin Cancer
Abstract
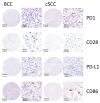
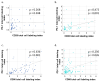
The establishment of immunotherapy applying immune checkpoint inhibitors (ICI) has provided an important new option for the treatment of solid malignant diseases. However, different tumor entities show dramatically different responses to this therapy. BCC responds worse to anti-PD-1 ICIs as compared to cSCC. Differential immune checkpoint expression could explain this discrepancy and, therefore, the aim of this study was to analyze activating and inhibitory immune checkpoints in cSCC and BCC tissues. Tissue microarrays of the invasive front as well as the tumor core of BCC and cSCC samples were used to evaluate PD-1, PD-L1, CD28, and CD86 expression and their topographic distribution profiles by chromogenic immunohistochemistry. QuPath was used to determine the labeling index. The expression of PD-1, PD-L1, and CD28 was significantly higher in both the tumor core and the invasive front of cSCC samples as compared to BCC (p < 0.001). In addition, the ratios of PD-L1/CD86 (p < 0.001) and CD28/CD86 (p < 0.001) were significantly higher in cSCC. The invasive front of both tumor entities showed higher expression levels of all immune markers compared to the tumor core in both tumor entities. The significantly higher expression of PD-1, PD-L1, and CD28 in cSCC, along with the predominance of the inhibitory ligand PD-L1 as compared to the activating CD86 in cSCC, provide a potential explanation for the better objective response rates to anti-PD-1 immunotherapy as compared to BCC. Furthermore, the predominant site of interaction between the immune system and the tumor was within the invasive front in both tumor types.
Keywords: BCC; CD28; CD86; IHC; NMSC; PD-1; PD-L1; TMA; TME; cSCC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Katalinic A., Kunze U., Schäfer T. Epidemiology of cutaneous melanoma and non-melanoma skin cancer in Schleswig-Holstein, Germany: Incidence, clinical subtypes, tumour stages and localization (epidemiology of skin cancer) Br. J. Dermatol. 2003;149:1200–1206. doi: 10.1111/j.1365-2133.2003.05554.x. - DOI - PubMed
-
- Ciazynska M., Kaminska-Winciorek G., Lange D., Lewandowski B., Reich A., Slawinska M., Pabianek M., Szczepaniak K., Hankiewicz A., Ulanska M., et al. Author Correction: The incidence and clinical analysis of non-melanoma skin cancer. Sci. Rep. 2021;11:15705. doi: 10.1038/s41598-021-94435-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

