A Flow Cytometry-Based Examination of the Mouse White Blood Cell Differential in the Context of Age and Sex
- PMID: 39329764
- PMCID: PMC11430320
- DOI: 10.3390/cells13181583
A Flow Cytometry-Based Examination of the Mouse White Blood Cell Differential in the Context of Age and Sex
Abstract
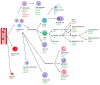
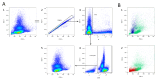
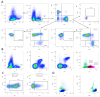
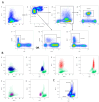
Analysis of the white blood cell differential as part of a flow cytometry-based approach is a common routine diagnostic tool used in clinics and research. For human blood, the methodological approach, suitable markers, and gating strategies are well-established. However, there is a lack of information regarding the mouse blood count. In this article, we deliver a fast and easy protocol for reprocessing mouse blood for the purpose of flow cytometric analysis, as well as suitable markers and gating strategies. We also present two possible applications: for the analysis of the whole blood count, with blood from a cardiac puncture, and for the analysis of a certain leukocyte subset at multiple time points in the framework of a mouse experiment, using blood from the facial vein. Additionally, we provide orientation values by applying the method to 3-month-old and 24-month-old male and female C57BL/6J mice. Our analyses demonstrate differences in the leukocyte fractions depending on age and sex. We discuss the influencing factors and limitations that can affect the results and that, therefore, need to be considered when applying this method. The present study fills the gap in the knowledge related to the rare information on flow cytometric analysis of mouse blood and, thus, lays the foundation for further investigations in this area.
Keywords: blood; blood sampling; immune cells; immune system; leukocyte subsets; leukocytes; mouse; mouse blood; mouse blood cells; reference values.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



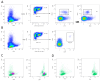



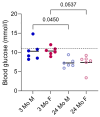
References
-
- Theml H., Diem H., Haferlach T. Taschenatlas der Hämatologie: Morphologische und klinische Diagnostik für die Praxis. Thieme; Stuttgart, NY, USA: 2002. 32 Tabellen, 5., vollst. überarb. Aufl.
-
- Bundesinstitut für Risikobewertung Zahlen zu den 2021 in Deutschland Verwendeten Versuchstieren. 2022. [(accessed on 15 September 2024)]. Available online: https://www.bf3r.de/de/verwendung_von_versuchstieren_im_berichtsjahr_202....
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

