Ultrasensitive Electrochemical Biosensor for Rapid Screening of Chemicals with Estrogenic Effect
- PMID: 39329811
- PMCID: PMC11430529
- DOI: 10.3390/bios14090436
Ultrasensitive Electrochemical Biosensor for Rapid Screening of Chemicals with Estrogenic Effect
Abstract
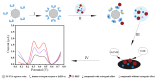
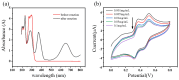
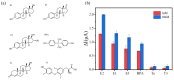
Estrogenic chemicals are widely distributed and structurally diverse. They primarily disrupt estrogen-related metabolism in animals or humans by mimicking the agonistic receptor effects of natural estrogens, thereby influencing the transcription of estrogen receptors to regulate their quantity and sensitivity. This disruption of estrogen-related metabolism can lead to estrogen-related effects, posing risks to biological health, emphasizing the urgent need for simple and effective methods to screen compounds with estrogenic effects. Herein, a new electrochemical biological effect biosensor based on human estrogen receptor α (hERα) is developed, which uses hERα as the biorecognition element and employs the electroactive horseradish peroxidase (HRP) labeled 17β-estradiol (E2) multifunctional conjugate HRP-E2 as the signal-boosting element and ligand competition agent. Based on the specific ligand-receptor interaction principle between the target and nuclear receptor, by allowing the test compound to compete with HRP-E2 conjugate for binding to hERα and testing the electrocatalytic signal of the conjugate that fails to bind to the hERα estrogen receptor, rapid screening and quantitative detection of chemical substances with estrogenic effect have been achieved. The biosensor shows a wide linear range of 40 pM to 40 nM with a detection limit of 17 pM (S/N = 3) for E2, and the detection limit is 2 orders of magnitude better than that of the previously reported sensors. The biosensor based on ligand-receptor binding can not only quantitatively analyze the typical estrogen E2, but also evaluate the relative estrogen effect strength of other estrogen compounds, which has good stability and selectivity. This electrochemical sensing platform displays its promising potential for rapid screening and quantitative detection of chemicals with estrogenic effects.
Keywords: electrochemical biosensor; endocrine disrupting chemicals (EDCs); estrogen; estrogenic activity evaluation; human estrogen receptor α.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Integral assessment of estrogenic potentials of sediment-associated samples. Part 1: The influence of salinity on the in vitro tests ELRA, E-Screen and YES.Environ Sci Pollut Res Int. 2008 Jan;15(1):75-83. doi: 10.1065/espr2007.06.429. Environ Sci Pollut Res Int. 2008. PMID: 18306891
-
Facile screening of potential xenoestrogens by an estrogen receptor-based reusable optical biosensor.Biosens Bioelectron. 2017 Nov 15;97:16-20. doi: 10.1016/j.bios.2017.05.026. Epub 2017 May 18. Biosens Bioelectron. 2017. PMID: 28549265
-
[Construction of a 17β-estradiol sensor based on a magnetic graphene oxide/aptamer separating material].Se Pu. 2025 Jan;43(1):87-95. doi: 10.3724/SP.J.1123.2024.06009. Se Pu. 2025. PMID: 39722625 Free PMC article. Chinese.
-
Estrogenic Endocrine Disrupting Chemicals Influencing NRF1 Regulated Gene Networks in the Development of Complex Human Brain Diseases.Int J Mol Sci. 2016 Dec 13;17(12):2086. doi: 10.3390/ijms17122086. Int J Mol Sci. 2016. PMID: 27983596 Free PMC article. Review.
-
Role of Estradiol Hormone in Human Life and Electrochemical Aptasensing of 17β-Estradiol: A Review.Biosensors (Basel). 2022 Dec 2;12(12):1117. doi: 10.3390/bios12121117. Biosensors (Basel). 2022. PMID: 36551086 Free PMC article. Review.
References
-
- Campbell C.G., Borglin S.E., Green F.B., Grayson A., Wozei E., Stringfellow W.T. Biologically directed environmental monitoring, fate, and transport of estrogenic endocrine disrupting compounds in water: A review. Chemosphere. 2006;65:1265–1280. doi: 10.1016/j.chemosphere.2006.08.003. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

