SARS-CoV-2 Infection Enhances Humoral Immune Response in Vaccinated Liver Transplant Recipients
- PMID: 39329897
- PMCID: PMC11428549
- DOI: 10.3390/antib13030078
SARS-CoV-2 Infection Enhances Humoral Immune Response in Vaccinated Liver Transplant Recipients
Abstract
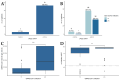
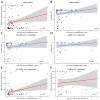
In the spring of 2020, the SARS-CoV-2 pandemic presented a formidable challenge to national and global healthcare systems. Immunocompromised individuals or those with relevant pre-existing conditions were particularly at risk of severe coronavirus disease 2019 (COVID-19). Thus, understanding the immunological processes in these patient groups is crucial for current research. This study aimed to investigate humoral immunity following vaccination and infection in liver transplant recipients. Humoral immunity analysis involved measuring IgG against the SARS-CoV-2 spike protein (anti-S IgG) and employing a surrogate virus neutralization test (sVNT) for assessing the hACE2 receptor-binding inhibitory capacity of antibodies. The study revealed that humoral immunity post-vaccination is well established, with positive results for anti-S IgG in 92.9% of the total study cohort. Vaccinated and SARS-CoV-2-infected patients exhibited significantly higher anti-S IgG levels compared to vaccinated, non-infected patients (18,590 AU/mL vs. 2320 AU/mL, p < 0.001). Additionally, a significantly elevated receptor-binding inhibitory capacity was observed in the cPassTMTM sVNT (96.4% vs. 91.8%, p = 0.004). Furthermore, a substantial enhancement of anti-S IgG levels (p = 0.034) and receptor-binding inhibition capacity (p < 0.001) was observed with an increasing interval post-transplantation (up to 30 years), calculated by generalized linear model analysis. In summary, fully vaccinated liver transplant recipients exhibit robust humoral immunity against SARS-CoV-2, which significantly intensifies following infection and with increasing time after transplantation. These findings should be considered for booster vaccination schemes for liver transplant recipients.
Keywords: SARS-CoV-2 infection; humoral immunity; liver transplantation; surrogate virus neutralization test; vaccination.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Ruether D.F., Schaub G.M., Duengelhoef P.M., Haag F., Brehm T.T., Fathi A., Wehmeyer M., Jahnke-Triankowski J., Mayer L., Hoffmann A., et al. SARS-CoV2-specific Humoral and T-cell Immune Response After Second Vaccination in Liver Cirrhosis and Transplant Patients. Clin. Gastroenterol. Hepatol. 2022;20:162–172.e9. doi: 10.1016/j.cgh.2021.09.003. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

