The ER Stress Induced in Human Neuroblastoma Cells Can Be Reverted by Lumacaftor, a CFTR Corrector
- PMID: 39329905
- PMCID: PMC11430679
- DOI: 10.3390/cimb46090553
The ER Stress Induced in Human Neuroblastoma Cells Can Be Reverted by Lumacaftor, a CFTR Corrector
Abstract
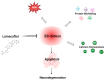
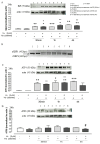
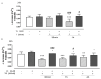
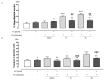
Most neurodegenerative diseases share a common etiopathogenesis, the accumulation of protein aggregates. An imbalance in homeostasis brought on by the buildup of misfolded proteins within the endoplasmic reticulum (ER) results in ER stress in the cell. Three distinct proteins found in the ER membrane-IRE1α, PERK, and ATF6-control the unfolded protein response (UPR), a signal transduction pathway that is triggered to restore normal physiological conditions. Buildup of misfolded proteins in ER lumen leads to a shunting of GRP78/BiP, thus triggering the UPR. PERK autophosphorylation leads to activation of ATF4, the transcription factor; finally, ATF6 activates the UPR's target genes, including GRP78/Bip. Accordingly, the UPR is a cellular reaction to an ER stress state that, if left unchecked for an extended period, results in apoptosis and irreversible damage. The identification of caspase 4, which is in the ER and is selectively activated by apoptotic stimuli caused by reticular stress, further demonstrated the connection between reticular stress and programed cell death. Moreover, oxidative stress and ER stress are linked. Oxidative stress is brought on by elevated quantities of radical oxygen species, both mitochondrial and cytosolic, that are not under the enzymatic regulation of superoxide dismutases, whose levels fall with increasing stress. Here, we evaluated the activity of Vx-809 (Lumacaftor), a drug used in cystic fibrosis, in SH-SY5Y neuronal cells, in which an ER stress condition was induced by Thapsigargin, to verify whether the drug could improve protein folding, suggesting its possible therapeutic use in proteinopathies, such as neurodegenerative diseases (NDs). Our data show that Vx-809 is involved in the significant reduction in protein produced under ER stress, particularly in the levels of Bip, ATF4, and ATF6 by Western blotting analysis, the reduction in ROS in the cytosol and mitochondria, and the reduction in the activation of the apoptotic pathway, measured by flow cytofluorimetry analysis and in restoring calcium homeostasis.
Keywords: ER stress; Lumacaftor; neuroblastoma cells; oxidative stress; protein misfolding.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Vx-809, a CFTR Corrector, Acts through a General Mechanism of Protein Folding and on the Inflammatory Process.Int J Mol Sci. 2023 Feb 20;24(4):4252. doi: 10.3390/ijms24044252. Int J Mol Sci. 2023. PMID: 36835664 Free PMC article.
-
Mechanism of the induction of endoplasmic reticulum stress by the anti-cancer agent, di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT): Activation of PERK/eIF2α, IRE1α, ATF6 and calmodulin kinase.Biochem Pharmacol. 2016 Jun 1;109:27-47. doi: 10.1016/j.bcp.2016.04.001. Epub 2016 Apr 6. Biochem Pharmacol. 2016. PMID: 27059255
-
Norlignans and phenolics from genus Curculigo protect corticosterone-injured neuroblastoma cells SH-SY5Y by inhibiting endoplasmic reticulum stress-mitochondria pathway.J Ethnopharmacol. 2022 Oct 5;296:115430. doi: 10.1016/j.jep.2022.115430. Epub 2022 Jun 2. J Ethnopharmacol. 2022. PMID: 35659626
-
Molecular signal networks and regulating mechanisms of the unfolded protein response.J Zhejiang Univ Sci B. 2017 Jan.;18(1):1-14. doi: 10.1631/jzus.B1600043. J Zhejiang Univ Sci B. 2017. PMID: 28070992 Free PMC article. Review.
-
Endoplasmic reticulum stress and its role in various neurodegenerative diseases.Brain Res. 2024 Mar 1;1826:148742. doi: 10.1016/j.brainres.2023.148742. Epub 2023 Dec 29. Brain Res. 2024. PMID: 38159591 Review.
Cited by
-
Modulation of Endoplasmic Reticulum Stress in Experimental Anti-Cancer Therapy.Int J Mol Sci. 2025 Jul 3;26(13):6407. doi: 10.3390/ijms26136407. Int J Mol Sci. 2025. PMID: 40650182 Free PMC article. Review.
-
Special Issue "Molecules at Play in Neurological Diseases".Curr Issues Mol Biol. 2025 Jul 30;47(8):600. doi: 10.3390/cimb47080600. Curr Issues Mol Biol. 2025. PMID: 40864754 Free PMC article.
References
-
- Ashraf G., Greig N., Khan T., Hassan I., Tabrez S., Shakil S., Sheikh I., Zaidi S., Akram M., Jabir N., et al. Protein Misfolding and Aggregation in Alzheimer’s Disease and Type 2 Diabetes Mellitus. CNS Neurol. Disord. Drug Targets. 2014;13:1280–1293. doi: 10.2174/1871527313666140917095514. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

