Outcomes of First Subsequent Taxane Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer Who Previously Received Docetaxel Intensification for Metastatic Castration-Sensitive Prostate Cancer
- PMID: 39330003
- PMCID: PMC11430621
- DOI: 10.3390/curroncol31090375
Outcomes of First Subsequent Taxane Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer Who Previously Received Docetaxel Intensification for Metastatic Castration-Sensitive Prostate Cancer
Abstract
Background: The management of advanced prostate cancer continues to evolve rapidly, particularly with the earlier use of survival-prolonging therapies in metastatic castration-sensitive prostate cancer (mCSPC). Though approved prior to the use of intensification therapy in mCSPC, taxane-based chemotherapies remain a relevant option for patients with metastatic castration-resistant prostate cancer (mCRPC). However, there is little evidence determining the outcomes of taxane chemotherapies as the first subsequent taxane (FST) in mCRPC pts who received docetaxel intensification (DI) in mCSPC. The purpose of this study is to compare outcomes between the survival-prolonging taxanes, docetaxel and cabazitaxel as FST after DI.
Methods: New patient consults seen at the Cross Cancer Institute from 1 July 2014 to 31 December 2020 were retrospectively reviewed. Pts were considered eligible if they received DI for mCSPC and then received either docetaxel or cabazitaxel in mCRPC. Variables of interest were collected from electronic medical records. The primary endpoint was ≥50% PSA response at 12 weeks relative to baseline for FST. Secondary endpoints included OS from mCSPC diagnosis, as well as PFS and OS from the FST start date. PSA responses were compared using the chi-squared test, and time-based endpoints were compared using the Kaplan-Meier method.
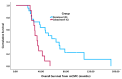
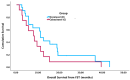
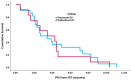
Results: In total, 34 pts were identified: docetaxel = 22 and cabazitaxel = 12 as FST. 91.2% of pts (docetaxel 95.5% vs. cabazitaxel 83.3%) received FST in 2nd line mCRPC. The median age at diagnosis (63.1 vs. 67.1 yrs, p = 0.236) and the median time to CRPC (18.6 vs. 14.2 mos, p = 0.079) were similar for docetaxel and cabazitaxel, respectively. The median time to FST (24.1 vs. 34.6 mos, p = 0.036) and OS from mCSPC diagnosis (30.9 vs. 52.7 mos, p = 0.002) were significantly shorter for pts receiving cabazitaxel vs. docetaxel. PSA responses occurred in 40.9% of pts treated with docetaxel compared to 25.0% treated with cabazitaxel (p = 0.645). There was no significant difference in median PFS (2.7 vs. 3.5 mos, p = 0.727) or median OS (11.4 vs. 8.1 mos, p = 0.132) from the time of FST for pts treated with docetaxel vs. cabazitaxel, respectively.
Conclusions: Both docetaxel and cabazitaxel demonstrated activity as FST after DI in mCSPC. Pts who received cabazitaxel had a shorter time to FST and OS from mCSPC. The reasons for this may reflect clinician preference for cabazitaxel in pts with aggressive or rapidly progressing disease. No difference was found in PSA response, PFS, or OS from FST with docetaxel compared to cabazitaxel. While limited by its retrospective nature and small sample size, this study suggests that docetaxel is active as FST despite treatment with DI in mCSPC.
Keywords: FST; docetaxel intensification; mCRPC; mCSPC; prostate cancer; taxanes.
Conflict of interest statement
Gabrielle Robin: No disclosures. Naveen S. Basappa: Astellas, AstraZeneca, Bayer, BMS, Eisai, EMD Serono, Ipsen, Janssen, Merck, Pfizer, Seagen, Takeda. Scott North: No disclosures. Sunita Ghosh: No disclosures. Michael Kolinsky: AstraZeneca, Astellas, Bayer, BMS, Eisai, EMD Serono, Ipsen, Janssen, Merck.
Figures
Similar articles
-
Plasma Cell-free DNA Concentration and Outcomes from Taxane Therapy in Metastatic Castration-resistant Prostate Cancer from Two Phase III Trials (FIRSTANA and PROSELICA).Eur Urol. 2018 Sep;74(3):283-291. doi: 10.1016/j.eururo.2018.02.013. Epub 2018 Feb 28. Eur Urol. 2018. PMID: 29500065 Free PMC article. Clinical Trial.
-
No significant impact of prior treatment profile with docetaxel on the efficacy of cabazitaxel in Japanese patients with metastatic castration-resistant prostate cancer.Med Oncol. 2017 Aug;34(8):141. doi: 10.1007/s12032-017-1005-3. Epub 2017 Jul 17. Med Oncol. 2017. PMID: 28718092 Clinical Trial.
-
No significant impact of patient age and prior treatment profile with docetaxel on the efficacy of cabazitaxel in patient with castration-resistant prostate cancer.Cancer Chemother Pharmacol. 2018 Dec;82(6):1061-1066. doi: 10.1007/s00280-018-3698-1. Epub 2018 Oct 3. Cancer Chemother Pharmacol. 2018. PMID: 30283980 Free PMC article.
-
Taxane-based Combination Therapies for Metastatic Prostate Cancer.Eur Urol Focus. 2019 May;5(3):369-380. doi: 10.1016/j.euf.2017.11.009. Epub 2017 Dec 21. Eur Urol Focus. 2019. PMID: 29275145 Review.
-
Current Evidence on Cabazitaxel for Prostate Cancer Therapy: A Narrative Review.Int J Urol. 2025 May;32(5):475-487. doi: 10.1111/iju.70019. Epub 2025 Feb 25. Int J Urol. 2025. PMID: 39996439 Free PMC article. Review.
Cited by
-
The efficacy and safety of cabazitaxel in the treatment of metastatic castration-resistant prostate cancer: a systematic review and network meta-analysis based on randomized controlled trials.Front Pharmacol. 2025 Jul 17;16:1586650. doi: 10.3389/fphar.2025.1586650. eCollection 2025. Front Pharmacol. 2025. PMID: 40746720 Free PMC article.
References
-
- Petrylak D.P., Tangen C.M., Hussain M.H., Lara P.N.J., Jones J.A., Taplin M.E., Burch P.A., Berry D., Moinpour C., Kohli M., et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. - DOI - PubMed
-
- James N.D., Sydes M.R., Clarke N.W., Mason M.D., Dearnaley D.P., Spears M.R., Ritchie A.W.S., Parker C.C., Russell J.M., Attard G., et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): Survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–1177. doi: 10.1016/S0140-6736(15)01037-5. - DOI - PMC - PubMed
-
- Kyriakopoulos C.E., Chen Y.-H., Carducci M.A., Liu G., Jarrard D.F., Hahn N.M., Shevrin D.H., Dreicer R., Hussain M., Eisenberger M., et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: Long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J. Clin. Oncol. 2018;36:1080–1087. doi: 10.1200/JCO.2017.75.3657. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

