Deceptive Measures of "Success" in Early Cancer Detection
- PMID: 39330008
- PMCID: PMC11431433
- DOI: 10.3390/curroncol31090380
Deceptive Measures of "Success" in Early Cancer Detection
Abstract
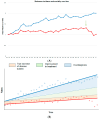
Early detection of cancer is considered a cornerstone of preventive medicine and is widely perceived as the gateway to reducing cancer deaths. Based on this assumption, large trials are currently underway to evaluate the accuracy of early detection tests. It is imperative, therefore, to set meaningful "success criteria" in early detection that reflect true improvements in health outcomes. This article discusses the pitfalls of measuring the success of early detection tests for cancer, particularly in the context of screening programs, and provides illustrative examples that demonstrate how commonly used metrics can be deceptive. Early detection can result in downstaging (favourable stage shift) when more early-stage cancers are diagnosed, even without reducing late-stage disease, potentially leading to overdiagnosis and overtreatment. Survival statistics, primarily cancer-specific survival, can be misleading due to lead time, where early detection simply extends the known duration of the disease without prolonging actual lifespan or improving overall survival. Additionally, the misuse of relative measures, such as proportions, ratios, and percentages, often make it impossible to ascertain the true benefit of a procedure and can distort the impact of screening as they are influenced by diagnostic practices, misleadingly improving perceived mortality reductions. Understanding these biases is crucial for accurately assessing the effectiveness of cancer detection methods and ensuring appropriate patient care.
Keywords: cancer; early diagnosis; overdiagnosis; stage distribution; survival.
Conflict of interest statement
The author declares no conflicts of interest.
Figures
References
-
- Wilson J.M.G., Jungner G. Principles and Practice of Screening for Disease. WHO Papers No 34. 1968. [(accessed on 26 February 2020)]. Available online: https://apps.who.int/iris/handle/10665/37650.
-
- Nicholson B.D., Oke J., Virdee P.S., Harris D.A., O’Doherty C., Park J.E., Hamady Z., Sehgal V., Millar A., Medley L., et al. Multi-cancer early detection test in symptomatic patients referred for cancer investigation in England and Wales (SYMPLIFY): A large-scale, observational cohort study. Lancet Oncol. 2023;24:733–743. doi: 10.1016/S1470-2045(23)00277-2. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

