Natural Regenerative Hydrogels for Wound Healing
- PMID: 39330149
- PMCID: PMC11431064
- DOI: 10.3390/gels10090547
Natural Regenerative Hydrogels for Wound Healing
Abstract
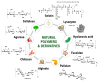
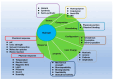
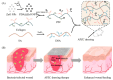
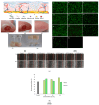
Regenerative hydrogels from natural polymers have come forth as auspicious materials for use in regenerative medicine, with interest attributed to their intrinsic biodegradability, biocompatibility, and ability to reassemble the extracellular matrix. This review covers the latest advances in regenerative hydrogels used for wound healing, focusing on their chemical composition, cross-linking mechanisms, and functional properties. Key carbohydrate polymers, including alginate, chitosan, hyaluronic acid, and polysaccharide gums, including agarose, carrageenan, and xanthan gum, are discussed in terms of their sources, chemical structures and specific properties suitable for regenerative applications. The review further explores the categorization of hydrogels based on ionic charge, response to physiological stimuli (i.e., pH, temperature) and particularized roles in wound tissue self-healing. Various methods of cross-linking used to enhance the mechanical and biological performance of these hydrogels are also examined. By highlighting recent innovations and ongoing challenges, this article intends to give a detailed understanding of natural hydrogels and their potential to revolutionize regenerative medicine and improve patient healing outcomes.
Keywords: antibacterial activity; biocompatibility; biodegradability; cross-linking mechanisms; extracellular matrix; glycosaminoglycans; polysaccharide gums; regenerative hydrogels; tissue engineering; wound healing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Revete A., Aparicio A., Cisterna B.A., Revete J., Luis L., Ibarra E., Segura González E.A., Molino J., Reginensi D. Advancements in the Use of Hydrogels for Regenerative Medicine: Properties and Biomedical Applications. Int. J. Biomater. 2022;1:3606765. doi: 10.1155/2022/3606765. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

