Papain Injection Creates a Nucleotomy-like Cavity for Testing Gels in Intervertebral Discs
- PMID: 39330173
- PMCID: PMC11430882
- DOI: 10.3390/gels10090571
Papain Injection Creates a Nucleotomy-like Cavity for Testing Gels in Intervertebral Discs
Abstract
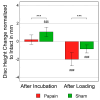
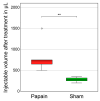
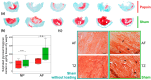
Biomaterials, such as hydrogels, have an increasingly important role in the development of regenerative approaches for the intervertebral disc. Since animal models usually resist biomaterial injection due to high intradiscal pressure, preclinical testing of the biomechanical performance of biomaterials after implantation remains difficult. Papain reduces the intradiscal pressure, creates cavities within the disc, and allows for biomaterial injections. But papain digestion needs time, and cadaver experiments that are limited to 24 h for measuring range of motion (ROM) cannot not be combined with papain digestion just yet. In this study, we successfully demonstrate a new organ culture approach, facilitating papain digestion to create cavities in the disc and the testing of ROM, neutral zone (NZ), and disc height. Papain treatment increased the ROM by up to 109.5%, extended NZ by up to 210.9%, and decreased disc height by 1.96 ± 0.74 mm. A median volume of 0.73 mL hydrogel could be injected after papain treatment, and histology revealed a strong loss of proteoglycans in the remaining nucleus tissue. Papain has the same biomechanical effects as known from nucleotomies or herniations and thus creates a disc model to study such pathologies in vitro. This new model can now be used to test the performance of biomaterials.
Keywords: biomechanics; chemonucleolysis; disc height; nucleus replacement; organ culture; papain; range of motion.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Vos T., Flaxman A.D., Naghavi M., Lozano R., Michaud C., Ezzati M., Shibuya K., Salomon J.A., Abdalla S., Aboyans V., et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. - DOI - PMC - PubMed
-
- Rodrigues-Pinto R., Richardson S.M., Hoyland J.A. An understanding of intervertebral disc development, maturation and cell phenotype provides clues to direct cell-based tissue regeneration therapies for disc degeneration. Eur. Spine J. 2014;23:1803–1814. doi: 10.1007/s00586-014-3305-z. - DOI - PubMed
-
- Cheung K.M., Karppinen J., Chan D., Ho D.W., Song Y.Q., Sham P., Cheah K.S., Leong J.C., Luk K.D. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine. 2009;34:934–940. doi: 10.1097/BRS.0b013e3181a01b3f. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

