Enhanced Production of High-Value Porphyrin Compound Heme by Metabolic Engineering Modification and Mixotrophic Cultivation of Synechocystis sp. PCC6803
- PMID: 39330259
- PMCID: PMC11433640
- DOI: 10.3390/md22090378
Enhanced Production of High-Value Porphyrin Compound Heme by Metabolic Engineering Modification and Mixotrophic Cultivation of Synechocystis sp. PCC6803
Abstract
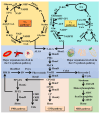
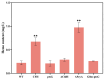
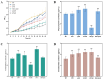
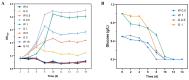
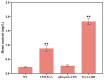
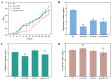
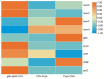
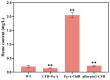
Heme, as an essential cofactor and source of iron for cells, holds great promise in various areas, e.g., food and medicine. In this study, the model cyanobacteria Synechocystis sp. PCC6803 was used as a host for heme synthesis. The heme synthesis pathway and its competitive pathway were modified to obtain an engineered cyanobacteria with high heme production, and the total heme production of Synechocystis sp. PCC6803 was further enhanced by the optimization of the culture conditions and the enhancement of mixotrophic ability. The co-expression of hemC, hemF, hemH, and the knockout of pcyA, a key gene in the heme catabolic pathway, resulted in a 3.83-fold increase in the heme production of the wild type, while the knockout of chlH, a gene encoding a Mg-chelatase subunit and the key enzyme of the chlorophyll synthesis pathway, resulted in a 7.96-fold increase in the heme production of the wild type; further increased to 2.05 mg/L, its heme production was 10.25-fold that of the wild type under optimized mixotrophic culture conditions. Synechocystis sp. PCC6803 has shown great potential as a cell factory for photosynthetic carbon sequestration for heme production. This study provides novel engineering targets and research directions for constructing microbial cell factories for efficient heme production.
Keywords: Synechocystis sp. PCC6803; cell factory; heme; metabolic transformation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Metabolic engineering of enhanced glycerol-3-phosphate synthesis to increase lipid production in Synechocystis sp. PCC 6803.Appl Microbiol Biotechnol. 2016 Jul;100(13):6091-101. doi: 10.1007/s00253-016-7521-9. Epub 2016 May 7. Appl Microbiol Biotechnol. 2016. PMID: 27154348
-
Photosynthetic production of itaconic acid in Synechocystis sp. PCC6803.J Biotechnol. 2015 Feb 10;195:43-5. doi: 10.1016/j.jbiotec.2014.12.016. Epub 2014 Dec 30. J Biotechnol. 2015. PMID: 25554635
-
Exploring the photosynthetic production capacity of sucrose by cyanobacteria.Metab Eng. 2013 Sep;19:17-25. doi: 10.1016/j.ymben.2013.05.001. Epub 2013 May 28. Metab Eng. 2013. PMID: 23721859
-
Synechocystis sp. PCC6803 metabolic models for the enhanced production of hydrogen.Crit Rev Biotechnol. 2015 Jun;35(2):184-98. doi: 10.3109/07388551.2013.829799. Epub 2013 Oct 3. Crit Rev Biotechnol. 2015. PMID: 24090244 Review.
-
Recent advances in microbial synthesis of free heme.Appl Microbiol Biotechnol. 2024 Dec;108(1):68. doi: 10.1007/s00253-023-12968-5. Epub 2024 Jan 9. Appl Microbiol Biotechnol. 2024. PMID: 38194135 Free PMC article. Review.
References
-
- Beas J.Z., Videira M.A., Saraiva L.M. Regulation of bacterial haem biosynthesis. Coord. Chem. Rev. 2022;452:214286. doi: 10.1016/j.ccr.2021.214286. - DOI
MeSH terms
Substances
Grants and funding
- 31972815/National Natural Science Foundation of China
- 42176124/National Natural Science Foundation of China
- ZR2019ZD17/Natural Science Foundation of Shandong Province
- ZR2020ZD23/Natural Science Foundation of Shandong Province
- ZR2021QD137/Natural Science Foundation of Shandong Province
- ZR2021MC051/Natural Science Foundation of Shandong Province
- ZR2023ZD30/Natural Science Foundation of Shandong Province
- BY2021KYQD18/Scientific Research Fund of Binzhou Medical University
- BY2021KYQD25/Scientific Research Fund of Binzhou Medical University
- BY2021KYQD28/Scientific Research Fund of Binzhou Medical University
- 2021RC007/Yantai Economic-Technological Development Area Innovation Pilot Project
LinkOut - more resources
Full Text Sources

