Study on Enzyme Activity and Metabolomics during Culture of Liquid Spawn of Floccularia luteovirens
- PMID: 39330377
- PMCID: PMC11433261
- DOI: 10.3390/jof10090618
Study on Enzyme Activity and Metabolomics during Culture of Liquid Spawn of Floccularia luteovirens
Abstract
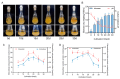
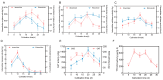
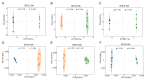
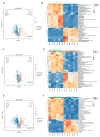
To comprehensively investigate the physiological characteristics and metabolic processes of the mycelium of Floccularia luteovirens (F. luteovirens), a wild edible fungus unique to the plateau region, we conducted an in-depth analysis of the mycelium enzyme activity and metabolites during different culture periods. The activity of seven enzymes all followed a trend of initially increasing and then decreasing. The intra- and extracellular activity peaks of three hydrolases-amylase, protease, and cellulase-all occurred on the 20th day, except for the extracellular amylase, which peaked on the 15th day. In contrast, the peak activity of laccase occurred on the 10th day. Moreover, three types of oxidoreductases in the mycelium (catalase (CAT), superoxide dismutase (SOD), and 2,3,5-triphenyltetrazolium chloride (TTC)-dehydrogenase (TTC-DH)) also exhibited significant changes in activity. CAT and SOD activity reached their maximum on the 20th day, whereas TTC-DH showed high activity on both the 10th and 20th days. Through a comprehensive assessment of the evolving trends of these physiological parameters, we determined that the optimal cultivation cycle for F. luteovirens liquid spawn is 20 days. An untargeted metabolomic analysis revealed that 3569 metabolites were detected in the F. luteovirens mycelium, including a variety of secondary metabolites and functional components, with terpenoids being particularly abundant, accounting for 148 types. By comparing three different culture stages (10 days, 20 days, and 30 days), 299, 291, and 381 metabolites, respectively, showed different accumulation patterns in the comparison groups of 10d vs. 20d, 20d vs. 30d, and 10d vs. 30d. These differential metabolites were primarily concentrated in carboxylic acids and their derivatives, fatty acyl groups, organic oxygen compounds, and lipid compounds. In addition, there were several amino acids whose abundance continued to grow during culturing. The metabolism of amino acids greatly affects mycelium growth and development. This research delineates the interplay between mycelium growth and metabolism, offering empirical support for a cultivation strategy for liquid F. luteovirens, and an exploration of its metabolites for potential applications.
Keywords: Floccularia luteovirens; enzyme activity; liquid spawn; metabolomics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Influence of Drying Methods on the Morphological Features, Microstructural Properties, and Antioxidant Performance of Floccularia luteovirens: A Metabolomic Analysis.J Fungi (Basel). 2025 Jan 19;11(1):78. doi: 10.3390/jof11010078. J Fungi (Basel). 2025. PMID: 39852497 Free PMC article.
-
Metabolomic Profiling of Floccularia luteovirens from Different Geographical Regions Proposes a Novel Perspective on Their Antioxidative Activities.Antioxidants (Basel). 2024 May 20;13(5):620. doi: 10.3390/antiox13050620. Antioxidants (Basel). 2024. PMID: 38790725 Free PMC article.
-
The Genomic and Transcriptomic Analyses of Floccularia luteovirens, a Rare Edible Fungus in the Qinghai-Tibet Plateau, Provide Insights into the Taxonomy Placement and Fruiting Body Formation.J Fungi (Basel). 2021 Oct 20;7(11):887. doi: 10.3390/jof7110887. J Fungi (Basel). 2021. PMID: 34829176 Free PMC article.
-
The Research Status and Prospects of Floccularia luteovirens: A Mycorrhizal Fungus with Edible Fruiting Bodies.J Fungi (Basel). 2023 Nov 1;9(11):1071. doi: 10.3390/jof9111071. J Fungi (Basel). 2023. PMID: 37998876 Free PMC article. Review.
-
The Molecular Mechanism of Yellow Mushroom (Floccularia luteovirens) Response to Strong Ultraviolet Radiation on the Qinghai-Tibet Plateau.Front Microbiol. 2022 Jun 20;13:918491. doi: 10.3389/fmicb.2022.918491. eCollection 2022. Front Microbiol. 2022. PMID: 35794915 Free PMC article.
Cited by
-
Influence of Drying Methods on the Morphological Features, Microstructural Properties, and Antioxidant Performance of Floccularia luteovirens: A Metabolomic Analysis.J Fungi (Basel). 2025 Jan 19;11(1):78. doi: 10.3390/jof11010078. J Fungi (Basel). 2025. PMID: 39852497 Free PMC article.
References
-
- Jiao L., Tao Y., Wang W., Mei L., Shao Y., Wang Q., Dang J. Chemical Constituents of Fruit Body of Armillaria luteo-virens. Chem. Nat. Compd. 2019;55:373–375. doi: 10.1007/s10600-019-02695-7. - DOI
-
- Bakratsas G., Polydera A., Katapodis P., Stamatis H. Recent trends in submerged cultivation of mushrooms and their application as a source of nutraceuticals and food additives. Future Foods. 2021;4:100086. doi: 10.1016/j.fufo.2021.100086. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

