In Vivo Efficacy of Rezafungin, Anidulafungin, Caspofungin, and Micafungin against Four Candida auris Clades in a Neutropenic Mouse Bloodstream Infection Model
- PMID: 39330378
- PMCID: PMC11433204
- DOI: 10.3390/jof10090617
In Vivo Efficacy of Rezafungin, Anidulafungin, Caspofungin, and Micafungin against Four Candida auris Clades in a Neutropenic Mouse Bloodstream Infection Model
Abstract
Objectives: Rezafungin is the first new drug approved to treat candidaemia and invasive candidiasis in more than 10 years. However, data are scant on the in vivo efficacy of rezafungin and the other three approved echinocandins against different Candida auris clades.
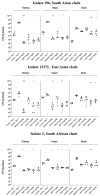
Methods: This study involved 10 isolates representing 4 C. auris clades: South Asian (n = 2), East Asian (n = 2), South African (n = 2), and South American (n = 4, including 2 environmental isolates). In the lethality experiment and fungal tissue burden experiment (kidney, heart, and brain), cyclophosphamide-treated BALB/c male mice were intravenously infected (107 and 8 × 106 colony-forming units [CFU]/mouse, respectively). A 20 mg/kg dose of rezafungin was administered on days 1, 3, and 6. Alternatively, beginning 24 h post-infection, mice received 3 mg/kg of caspofungin, 5 mg/kg of micafungin, or 5 mg/kg of anidulafungin once daily for 6 days.
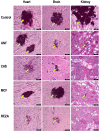
Results: Regardless of isolate and clade, all echinocandin regimens improved survival after 21 days (p = 0.0041 to p < 0.0001). All echinocandins frequently produced >3-log mean CFU/g decreases in the fungal kidney and heart burdens, although some of these decreases were not statistically significant. Rezafungin, regardless of clade, produced 3-5 and 2-4 log CFU/g decreases in the kidney and heart burdens, respectively. Echinocandins did not inhibit fungal growth in the brain. Histopathological examination performed on day 7 showed no fungal cells in the heart and kidneys of rezafungin-treated mice and to a lesser extent, caspofungin-treated mice, regardless of the clinical isolate. All echinocandin-treated mice showed medium and/or large foci of fungal cells in their cerebrum or cerebellum.
Conclusions: Regardless of the C. auris clade, rezafungin activity in vivo was comparable to or improved over that of the three previously approved echinocandins.
Keywords: Candida auris; echinocandins; murine model.
Conflict of interest statement
László Majoros received conference travel grants from Cidara, MSD, Astellas and Pfizer. J.B. Locke is an employee and shareholder of Cidara Therapeutics, Inc. All other authors report no conflicts of interest.
Figures


Similar articles
-
Comparison of In Vitro Killing Activity of Rezafungin, Anidulafungin, Caspofungin, and Micafungin against Four Candida auris Clades in RPMI-1640 in the Absence and Presence of Human Serum.Microorganisms. 2021 Apr 16;9(4):863. doi: 10.3390/microorganisms9040863. Microorganisms. 2021. PMID: 33923783 Free PMC article.
-
Evaluation of the efficacy of rezafungin, a novel echinocandin, in the treatment of disseminated Candida auris infection using an immunocompromised mouse model.J Antimicrob Chemother. 2018 Aug 1;73(8):2085-2088. doi: 10.1093/jac/dky153. J Antimicrob Chemother. 2018. PMID: 29897469 Free PMC article.
-
Relative Frequency of Paradoxical Growth and Trailing Effect with Caspofungin, Micafungin, Anidulafungin, and the Novel Echinocandin Rezafungin against Candida Species.J Fungi (Basel). 2020 Aug 17;6(3):136. doi: 10.3390/jof6030136. J Fungi (Basel). 2020. PMID: 32824464 Free PMC article.
-
Echinocandin antifungal drugs in fungal infections: a comparison.Drugs. 2011 Jan 1;71(1):11-41. doi: 10.2165/11585270-000000000-00000. Drugs. 2011. PMID: 21175238 Review.
-
Rezafungin: A Review in Invasive Candidiasis.Drugs. 2025 Mar;85(3):415-423. doi: 10.1007/s40265-024-02134-0. Epub 2025 Feb 6. Drugs. 2025. PMID: 39913021 Review.
Cited by
-
Tolerance and heteroresistance to echinocandins in Candida auris: conceptual issues, clinical implications, and outstanding questions.mSphere. 2025 May 27;10(5):e0016125. doi: 10.1128/msphere.00161-25. Epub 2025 Apr 16. mSphere. 2025. PMID: 40237528 Free PMC article. Review.
-
Activity of rezafungin against Candida auris.J Antimicrob Chemother. 2025 Jun 3;80(6):1482-1493. doi: 10.1093/jac/dkaf124. J Antimicrob Chemother. 2025. PMID: 40304092 Free PMC article. Review.
References
-
- World Health Organization . WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. World Health Organization; Geneva, Switzerland: 2022.
-
- Lockhart S.R., Etienne K.A., Vallabhaneni S., Farooqi J., Chowdhary A., Govender N.P., Colombo A.L., Calvo B., Cuomo C.A., Desjardins C.A., et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin. Infect. Dis. 2017;64:134–140. doi: 10.1093/cid/ciw691. - DOI - PMC - PubMed
-
- Pappas P.G., Kauffman C.A., Andes D.R., Clancy C.J., Marr K.A., Ostrosky-Zeichner L., Reboli A.C., Schuster M.G., Vazquez J.A., Walsh T.J., et al. Clinical practice guideline for the management of candidiasis: Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016;62:e1–e50. doi: 10.1093/cid/civ933. - DOI - PMC - PubMed
-
- CDC [(accessed on 1 July 2024)]; Available online: https://www.cdc.gov/candida-auris/hcp/laboratories/antifungal-susceptibi....
Grants and funding
LinkOut - more resources
Full Text Sources

