Potential Bioactivities, Chemical Composition, and Conformation Studies of Exopolysaccharide-Derived Aspergillus sp. Strain GAD7
- PMID: 39330418
- PMCID: PMC11432975
- DOI: 10.3390/jof10090659
Potential Bioactivities, Chemical Composition, and Conformation Studies of Exopolysaccharide-Derived Aspergillus sp. Strain GAD7
Abstract
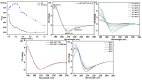
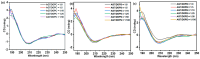
This research identified a marine fungal isolate, Aspergillus sp. strain GAD7, which produces an acidic and sulfated extracellular polysaccharide (EPS) with notable anticoagulant and antioxidant properties. Six fungal strains from the Egyptian Mediterranean Sea were screened for EPS production, with Aspergillus sp. strain GAD7 (EPS-AG7) being the most potent, yielding ~5.19 ± 0.017 g/L. EPS-AG7 was characterized using UV-Vis and FTIR analyses, revealing high carbohydrate (87.5%) and sulfate (24%) contents. HPLC and GC-MS analyses determined that EPS-AG7 is a heterogeneous acidic polysaccharide with an average molecular weight (Mw¯) of ~7.34 × 103 Da, composed of mannose, glucose, arabinose, galacturonic acid, galactose, and lyxose in a molar ratio of 6.6:3.9:1.8:1.3:1.1:1.0, linked through α- and β-glycosidic linkages as confirmed by NMR analysis. EPS-AG7 adopted a triple helix-like conformation, as evidenced by UV-Vis (Congo Red experiment) and circular dichroism (CD) studies. This helical arrangement demonstrated stability under various experimental conditions, including concentration, ionic strength, temperature, and lipid interactions. EPS-AG7 exhibited significant anticoagulant activity, doubling blood coagulation time at a concentration of 3.0 mg/mL, and showed significant antioxidant activity, with scavenging activities reaching up to 85.90% and 58.64% in DPPH and ABTS+ assays at 5.0 mg/mL, and EC50 values of 1.40 mg/mL and 3.80 mg/mL, respectively. These findings highlight the potential of EPS-AG7 for therapeutic applications due to its potent biological activities.
Keywords: Aspergillus sp.; biological activities; circular dichroism; exopolysaccharide; helix-like conformation; structural analysis.
Conflict of interest statement
The authors declare they have no conflicts of interest.
Figures




Similar articles
-
[Physicochemical property and antioxidant activity of exopolysaccharide produced by endophytic fungal Fusarium redolens 6WBY3 isolated from Fritillaria unibracteata var. wabuensis].Wei Sheng Wu Xue Bao. 2017 Feb 4;57(2):240-53. Wei Sheng Wu Xue Bao. 2017. PMID: 29750487 Chinese.
-
Polysaccharides from Exocarpium Citri Grandis: Graded Ethanol Precipitation, Structural Characterization, Inhibition of α-Glucosidase Activity, Anti-Oxidation, and Anti-Glycation Potentials.Foods. 2025 Feb 25;14(5):791. doi: 10.3390/foods14050791. Foods. 2025. PMID: 40077493 Free PMC article.
-
Purification, characterization and bioactivity of exopolysaccharides produced by Lactobacillus plantarum KX041.Int J Biol Macromol. 2019 May 1;128:480-492. doi: 10.1016/j.ijbiomac.2019.01.117. Epub 2019 Jan 23. Int J Biol Macromol. 2019. PMID: 30682478
-
Production and Characterization of Exopolysaccharide From Newly Isolated Marine Probiotic Lactiplantibacillus plantarum EI6 With in vitro Wound Healing Activity.Front Microbiol. 2022 May 13;13:903363. doi: 10.3389/fmicb.2022.903363. eCollection 2022. Front Microbiol. 2022. PMID: 35668753 Free PMC article.
-
Characterization of Lactic Acid Bacterium Exopolysaccharide, Biological, and Nutritional Evaluation of Probiotic Formulated Fermented Coconut Beverage.Int J Food Sci. 2024 Sep 2;2024:8923217. doi: 10.1155/2024/8923217. eCollection 2024. Int J Food Sci. 2024. PMID: 39257841 Free PMC article. Review.
Cited by
-
Unveiling the rhizosphere microbiome of Dendrobium: mechanisms, microbial interactions, and implications for sustainable agriculture.Front Microbiol. 2025 Jan 29;16:1531900. doi: 10.3389/fmicb.2025.1531900. eCollection 2025. Front Microbiol. 2025. PMID: 39944638 Free PMC article. Review.
References
-
- Suryawanshi N., Naik S., Jujjawarapu S.E. Exopolysaccharides and Their Applications in Food Processing Industries. Food Sci. Appl. Biotechnol. 2022;5:22–44. doi: 10.30721/fsab2022.v5.i1.165. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

