Functional Muffins Exert Bifidogenic Effects along with Highly Product-Specific Effects on the Human Gut Microbiota Ex Vivo
- PMID: 39330504
- PMCID: PMC11433953
- DOI: 10.3390/metabo14090497
Functional Muffins Exert Bifidogenic Effects along with Highly Product-Specific Effects on the Human Gut Microbiota Ex Vivo
Abstract
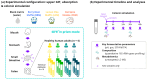
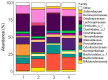
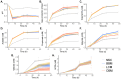
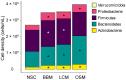
GoodBiome™ Foods are functional foods containing a probiotic (Bacillus subtilis HU58™) and prebiotics (mainly inulin). Their effects on the human gut microbiota were assessed using ex vivo SIFR® technology, which has been validated to provide clinically predictive insights. GoodBiome™ Foods (BBM/LCM/OSM) were subjected to oral, gastric, and small intestinal digestion/absorption, after which their impact on the gut microbiome of four adults was assessed (n = 3). All GoodBiome™ Foods boosted health-related SCFA acetate (+13.1/14.1/13.8 mM for BBM/LCM/OSM), propionate (particularly OSM; +7.4/7.5/8.9 mM for BBM/LCM/OSM) and butyrate (particularly BBM; +2.6/2.1/1.4 mM for BBM/LCM/OSM). This is related to the increase in Bifidobacterium species (B. catenulatum, B. adolescentis, B. pseudocatenulatum), Coprococcus catus and Bacteroidetes members (Bacteroides caccae, Phocaeicola dorei, P. massiliensis), likely mediated via inulin. Further, the potent propionogenic potential of OSM related to increased Bacteroidetes members known to ferment oats (s key ingredient of OSM), while the butyrogenic potential of BBM related to a specific increase in Anaerobutyricum hallii, a butyrate producer specialized in the fermentation of erythritol (key ingredient of BBM). In addition, OSM/BBM suppressed the pathogen Clostridioides difficile, potentially due to inclusion of HU58™ in GoodBiome™ Foods. Finally, all products enhanced a spectrum of metabolites well beyond SCFA, including vitamins (B3/B6), essential amino acids, and health-related metabolites such as indole-3-propionic acid. Overall, the addition of specific ingredients to complex foods was shown to specifically modulate the gut microbiome, potentially contributing to health benefits. Noticeably, our findings contradict a recent in vitro study, underscoring the critical role of employing a physiologically relevant digestion/absorption procedure for a more accurate evaluation of the microbiome-modulating potential of complex foods.
Keywords: berry blast muffin (BBM); functional foods; lemon chia muffin (LCM); oat spice mookie (OSM); prebiotics; probiotics; short-chain fatty acid (SCFA); systemic intestinal fermentation research (SIFR).
Conflict of interest statement
T.B., K.K., and M.G. are employees of Microbiome Labs. While the authors participated in the design of the study, the interpretation of the data, and the revision of the manuscript, they did not participate in the collection and analysis of data. S.D., P.V.d.A., J.P., L.D.V., and A.B. are employees of Cryptobiotix SA. Finally, S.B. received payment from Microbiome Labs to write the first draft of the manuscript.
Figures


Similar articles
-
HMOs Exert Marked Bifidogenic Effects on Children's Gut Microbiota Ex Vivo, Due to Age-Related Bifidobacterium Species Composition.Nutrients. 2023 Mar 30;15(7):1701. doi: 10.3390/nu15071701. Nutrients. 2023. PMID: 37049541 Free PMC article.
-
Serum-Derived Bovine Immunoglobulin Promotes Barrier Integrity and Lowers Inflammation for 24 Human Adults Ex Vivo.Nutrients. 2024 May 23;16(11):1585. doi: 10.3390/nu16111585. Nutrients. 2024. PMID: 38892520 Free PMC article.
-
Bridging preclinical and clinical gut microbiota research using the ex vivo SIFR® technology.Front Microbiol. 2023 Apr 14;14:1131662. doi: 10.3389/fmicb.2023.1131662. eCollection 2023. Front Microbiol. 2023. PMID: 37187538 Free PMC article.
-
Recent advances in developing butyrogenic functional foods to promote gut health.Crit Rev Food Sci Nutr. 2024;64(13):4410-4431. doi: 10.1080/10408398.2022.2142194. Epub 2022 Nov 4. Crit Rev Food Sci Nutr. 2024. PMID: 36330804 Review.
-
Effectiveness of probiotics, prebiotics, and prebiotic-like components in common functional foods.Compr Rev Food Sci Food Saf. 2020 Jul;19(4):1908-1933. doi: 10.1111/1541-4337.12565. Epub 2020 May 26. Compr Rev Food Sci Food Saf. 2020. PMID: 33337097 Review.
Cited by
-
Lactoferrin-osteopontin complexes: insights into intestinal organoid bioavailability and gut microbiota modulation.Commun Biol. 2025 Jul 8;8(1):1017. doi: 10.1038/s42003-025-08460-7. Commun Biol. 2025. PMID: 40629168 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

