Cerebral Vascular Toxicity after Developmental Exposure to Arsenic (As) and Lead (Pb) Mixtures
- PMID: 39330552
- PMCID: PMC11435665
- DOI: 10.3390/toxics12090624
Cerebral Vascular Toxicity after Developmental Exposure to Arsenic (As) and Lead (Pb) Mixtures
Abstract
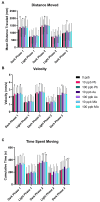
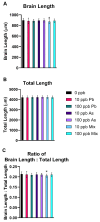
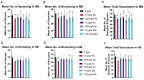
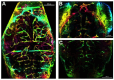
Arsenic (As) and lead (Pb) are environmental pollutants found in common sites linked to similar adverse health effects. This study determined driving factors of neurotoxicity on the developing cerebral vasculature with As and Pb mixture exposures. Cerebral vascular toxicity was evaluated at mixture concentrations of As and Pb representing human exposures levels (10 or 100 parts per billion; ppb; µg/L) in developing zebrafish by assessing behavior, morphology, and gene expression. In the visual motor response assay, hyperactivity was observed in all three outcomes in dark phases in larvae with exposure (1-120 h post fertilization, hpf) to 10 ppb As, 10 ppb Pb, or 10 ppb mix treatment. Time spent moving exhibited hyperactivity in dark phases for 100 ppb As and 100 ppb mix treatment groups only. A decreased brain length and ratio of brain length to total length in the 10 ppb mix group was measured with no alterations in other treatment groups or other endpoints (i.e., total larval length, head length, or head width). Alternatively, measurements of cerebral vasculature in the midbrain and cerebellum uncovered decreased total vascularization at 72 hpf in all treatment groups in the mesencephalon and in all treatment groups, except the 100 ppb Pb and 10 ppb As groups, in the cerebellum. In addition, decreased sprouting and branching occurred in the mesencephalon, while only decreased branching was measured in the cerebellum. The 10 ppb Pb group showed several cerebral vasculature modifications that were aligned with a specific gene expression alteration pattern different from other treatment groups. Additionally, the 100 ppb As group drove gene alterations, along with several other endpoints, for changes observed in the 100 ppb mix treatment group. Perturbations assessed in this study displayed non-linear concentration-responses, which are important to consider in environmental health outcomes for As and Pb neurotoxicity.
Keywords: arsenic; behavior; cerebral vasculature; lead; metal; neurotoxicity; zebrafish.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Joint Action Toxicity of Arsenic (As) and Lead (Pb) Mixtures in Developing Zebrafish.Biomolecules. 2022 Dec 8;12(12):1833. doi: 10.3390/biom12121833. Biomolecules. 2022. PMID: 36551261 Free PMC article.
-
Developmental atrazine exposure in zebrafish produces the same major metabolites as mammals along with altered behavioral outcomes.Neurotoxicol Teratol. 2021 May-Jun;85:106971. doi: 10.1016/j.ntt.2021.106971. Epub 2021 Mar 10. Neurotoxicol Teratol. 2021. PMID: 33713789 Free PMC article.
-
Adverse developmental impacts in progeny of zebrafish exposed to the agricultural herbicide atrazine during embryogenesis.Environ Int. 2023 Oct;180:108213. doi: 10.1016/j.envint.2023.108213. Epub 2023 Sep 24. Environ Int. 2023. PMID: 37774458 Free PMC article.
-
Zebrafish as a model for investigating developmental lead (Pb) neurotoxicity as a risk factor in adult neurodegenerative disease: a mini-review.Neurotoxicology. 2014 Jul;43:57-64. doi: 10.1016/j.neuro.2014.03.008. Epub 2014 Mar 31. Neurotoxicology. 2014. PMID: 24698670 Review.
-
Neurotoxicity of Metal Mixtures.Adv Neurobiol. 2017;18:227-265. doi: 10.1007/978-3-319-60189-2_12. Adv Neurobiol. 2017. PMID: 28889271 Review.
References
-
- Zhu X., Victor T.W., Ambi A., Sullivan J.K., Hatfield J., Xu F., Miller L.M., E Van Nostrand W. Copper accumulation and the effect of chelation treatment on cerebral amyloid angiopathy compared to parenchymal amyloid plaques. Metallomics. 2020;12:539–546. doi: 10.1039/c9mt00306a. - DOI - PMC - PubMed
-
- Hirooka T., Fujiwara Y., Inoue S., Shinkai Y., Yamamoto C., Satoh M., Yasutake A., Eto K., Kaji T. Suppression of fibroblast growth factor-2 expression: Possible mechanism underlying methylmercury-induced inhibition of the repair of wounded monolayers of cultured human brain microvascular endothelial cells. J. Toxicol. Sci. 2009;34:433–439. doi: 10.2131/jts.34.433. - DOI - PubMed
-
- Tobwala S., Wang H.-J., Carey J.W., Banks W.A., Ercal N. Effects of Lead and Cadmium on Brain Endothelial Cell Survival, Monolayer Permeability, and Crucial Oxidative Stress Markers in an in Vitro Model of the Blood-Brain Barrier. Toxics. 2014;2:258–275. doi: 10.3390/toxics2020258. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

