Multi-Walled Carbon Nanotubes Accelerate Leukaemia Development in a Mouse Model
- PMID: 39330574
- PMCID: PMC11435454
- DOI: 10.3390/toxics12090646
Multi-Walled Carbon Nanotubes Accelerate Leukaemia Development in a Mouse Model
Erratum in
-
Correction: Wang et al. Multi-Walled Carbon Nanotubes Accelerate Leukaemia Development in a Mouse Model. Toxics 2024, 12, 646.Toxics. 2024 Nov 18;12(11):823. doi: 10.3390/toxics12110823. Toxics. 2024. PMID: 39591013 Free PMC article.
Abstract
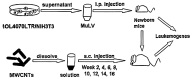
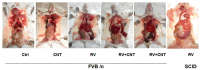
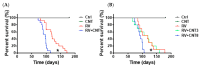
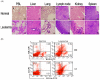
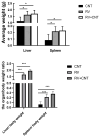
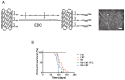
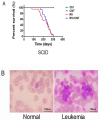
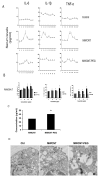
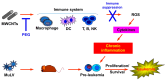
Inflammation is associated with an increased risk of developing various cancers in both animals and humans, primarily solid tumors but also myeloproliferative neoplasms (MPNs), myelodysplastic syndromes (MDS), and acute myeloid leukemia (AML). Multi-walled carbon nanotubes (MWCNTs), a type of carbon nanotubes (CNTs) increasingly used in medical research and other fields, are leading to a rising human exposure. Our study demonstrated that exposing mice to MWCNTs accelerated the progression of spontaneous MOL4070LTR virus-induced leukemia. Additionally, similar exposures elevated pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α and induced reactive oxygen species (ROS) in a murine macrophage cell line. These effects were significantly reduced in immunodeficient mice and when mice were treated with methoxypolyethylene glycol amine (PEG)-modified MWCNTs. These findings underscore the necessity of evaluating the safety of MWCNTs, particularly for those with hematologic cancers.
Keywords: MOL4070LTR; PEG; inflammation; leukemia; multi-walled carbon nanotubes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Surface modification of multiwall carbon nanotubes determines the pro-inflammatory outcome in macrophage.J Hazard Mater. 2015 Mar 2;284:73-82. doi: 10.1016/j.jhazmat.2014.11.013. Epub 2014 Nov 15. J Hazard Mater. 2015. PMID: 25463220
-
Atomic layer deposition coating of carbon nanotubes with aluminum oxide alters pro-fibrogenic cytokine expression by human mononuclear phagocytes in vitro and reduces lung fibrosis in mice in vivo.PLoS One. 2014 Sep 12;9(9):e106870. doi: 10.1371/journal.pone.0106870. eCollection 2014. PLoS One. 2014. PMID: 25216247 Free PMC article.
-
Role of inflammation in the malignant transformation of pleural mesothelial cells induced by multi-walled carbon nanotubes.Nanotoxicology. 2020 Sep;14(7):947-967. doi: 10.1080/17435390.2020.1777477. Epub 2020 Jun 23. Nanotoxicology. 2020. PMID: 32574520
-
Carbon nanotubes for delivery of small molecule drugs.Adv Drug Deliv Rev. 2013 Dec;65(15):1964-2015. doi: 10.1016/j.addr.2013.08.005. Epub 2013 Aug 14. Adv Drug Deliv Rev. 2013. PMID: 23954402 Review.
-
Pleural translocation and lesions by pulmonary exposed multi-walled carbon nanotubes.J Toxicol Pathol. 2020 Jul;33(3):145-151. doi: 10.1293/tox.2019-0075. Epub 2020 Mar 13. J Toxicol Pathol. 2020. PMID: 32764839 Free PMC article. Review.
Cited by
-
Nanotoxicology: developments and new insights.Nanomedicine (Lond). 2025 Jan;20(2):225-241. doi: 10.1080/17435889.2024.2443385. Epub 2024 Dec 26. Nanomedicine (Lond). 2025. PMID: 39723590 Review.
-
Therapeutic innovations: targeting ROS production in AML with natural and synthetic compounds.Naunyn Schmiedebergs Arch Pharmacol. 2025 Mar 31. doi: 10.1007/s00210-025-04054-6. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40163149 Review.
-
Correction: Wang et al. Multi-Walled Carbon Nanotubes Accelerate Leukaemia Development in a Mouse Model. Toxics 2024, 12, 646.Toxics. 2024 Nov 18;12(11):823. doi: 10.3390/toxics12110823. Toxics. 2024. PMID: 39591013 Free PMC article.
References
-
- Shi H.K., Carter S., Haines N.L. Harvesting electrical energy from carbon nanotube yarn twist. Science. 2017;357:773–778. - PubMed
-
- Lima M.D., Li N., Jung de Andrade M., Fang S., Oh J., Spinks G.M., Kozlov M.E., Haines C.S., Suh D., Foroughi J., et al. Electrically, Chemically, and Photonically Powered Torsional and Tensile Actuation of Hybrid Carbon Nanotube Yarn Muscles. Science. 2012;338:928–932. doi: 10.1126/science.1226762. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

