A High-Throughput Method for Quantifying Drosophila Fecundity
- PMID: 39330586
- PMCID: PMC11436201
- DOI: 10.3390/toxics12090658
A High-Throughput Method for Quantifying Drosophila Fecundity
Abstract
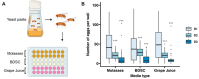
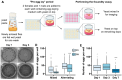
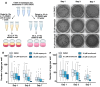
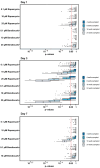
The fruit fly, Drosophila melanogaster, is an experimentally tractable model system that has recently emerged as a powerful "new approach methodology" (NAM) for chemical safety testing. As oogenesis is well conserved at the molecular and cellular level, measurements of Drosophila fecundity can be useful for identifying chemicals that affect reproductive health across species. However, standard Drosophila fecundity assays have been difficult to perform in a high-throughput manner because experimental factors such as the physiological state of the flies and environmental cues must be carefully controlled to achieve consistent results. In addition, exposing flies to a large number of different experimental conditions (such as chemical additives in the diet) and manually counting the number of eggs laid to determine the impact on fecundity is time-consuming. We have overcome these challenges by combining a new multiwell fly culture strategy with a novel 3D-printed fly transfer device to rapidly and accurately transfer flies from one plate to another, the RoboCam, a low-cost, custom-built robotic camera to capture images of the wells automatically, and an image segmentation pipeline to automatically identify and quantify eggs. We show that this method is compatible with robust and consistent egg laying throughout the assay period and demonstrate that the automated pipeline for quantifying fecundity is very accurate (r2 = 0.98 for the correlation between the automated egg counts and the ground truth). In addition, we show that this method can be used to efficiently detect the effects on fecundity induced by dietary exposure to chemicals. Taken together, this strategy substantially increases the efficiency and reproducibility of high-throughput egg-laying assays that require exposing flies to multiple different media conditions.
Keywords: Drosophila; oogenesis; reproductive toxicology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Update of
-
A high-throughput method for quantifying Drosophila fecundity.bioRxiv [Preprint]. 2024 Mar 30:2024.03.27.587093. doi: 10.1101/2024.03.27.587093. bioRxiv. 2024. Update in: Toxics. 2024 Sep 09;12(9):658. doi: 10.3390/toxics12090658. PMID: 38585877 Free PMC article. Updated. Preprint.
Similar articles
-
A high-throughput method for quantifying Drosophila fecundity.bioRxiv [Preprint]. 2024 Mar 30:2024.03.27.587093. doi: 10.1101/2024.03.27.587093. bioRxiv. 2024. Update in: Toxics. 2024 Sep 09;12(9):658. doi: 10.3390/toxics12090658. PMID: 38585877 Free PMC article. Updated. Preprint.
-
Evolutionary shifts in taste coding in the fruit pest Drosophila suzukii.Elife. 2021 Feb 22;10:e64317. doi: 10.7554/eLife.64317. Elife. 2021. PMID: 33616529 Free PMC article.
-
A model-based high throughput method for fecundity estimation in fruit fly studies.Fly (Austin). 2018;12(3-4):183-190. doi: 10.1080/19336934.2018.1562267. Epub 2018 Dec 30. Fly (Austin). 2018. PMID: 30580661 Free PMC article.
-
Turning food into eggs: insights from nutritional biology and developmental physiology of Drosophila.Curr Opin Insect Sci. 2019 Feb;31:49-57. doi: 10.1016/j.cois.2018.08.006. Epub 2018 Aug 30. Curr Opin Insect Sci. 2019. PMID: 31109673 Review.
-
Fecundity and life-history strategies in marine invertebrates.Adv Mar Biol. 2002;43:87-170. doi: 10.1016/s0065-2881(02)43004-0. Adv Mar Biol. 2002. PMID: 12154615 Review.
Cited by
-
In Silico and In Vivo Investigation of the Anti-Hyperglycemic Effects of Caffeic Acid.ACS Omega. 2025 Apr 1;10(14):14052-14062. doi: 10.1021/acsomega.4c11062. eCollection 2025 Apr 15. ACS Omega. 2025. PMID: 40256540 Free PMC article.
References
-
- Stucki A.O., Barton-Maclaren T.S., Bhuller Y., Henriquez J.E., Henry T.R., Hirn C., Miller-Holt J., Nagy E.G., Perron M.M., Ratzlaff D.E., et al. Use of New Approach Methodologies (NAMs) to Meet Regulatory Requirements for the Assessment of Industrial Chemicals and Pesticides for Effects on Human Health. Front. Toxicol. 2022;4:964553. doi: 10.3389/ftox.2022.964553. - DOI - PMC - PubMed
-
- Wambaugh J.F., Bare J.C., Carignan C.C., Dionisio K.L., Dodson R.E., Jolliet O., Liu X., Meyer D.E., Newton S.R., Phillips K.A., et al. New Approach Methodologies for Exposure Science. Curr. Opin. Toxicol. 2019;15:76–92. doi: 10.1016/j.cotox.2019.07.001. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

