In Vitro Hepatic Clearance Evaluations of Per- and Polyfluoroalkyl Substances (PFAS) across Multiple Structural Categories
- PMID: 39330600
- PMCID: PMC11435625
- DOI: 10.3390/toxics12090672
In Vitro Hepatic Clearance Evaluations of Per- and Polyfluoroalkyl Substances (PFAS) across Multiple Structural Categories
Abstract
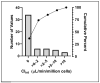
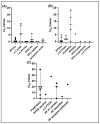
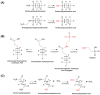
Toxicokinetic (TK) assays and in vitro-in vivo extrapolation (IVIVE) models are New Approach Methods (NAMs) used to translate in vitro points of departure to exposure estimates required to reach equivalent blood concentrations. Per- and polyfluoroalkyl substances (PFAS) are a large chemical class with wide-ranging industrial applications for which only limited toxicity data are available for human health evaluation. To address the lack of TK data, a pooled primary human hepatocyte suspension model was used with targeted liquid chromatography-mass spectrometry to investigate substrate depletion for 54 PFAS. A median value of 4.52 μL/(min x million cells) was observed across those that showed significant clearance, with 35 displaying no substrate depletion. Bayesian modeling propagated uncertainty around clearance values for use in IVIVE models. Structural evaluations showed the fluorotelomer carboxylic acids were the only PFAS carboxylates showing appreciable clearance, and per- and polyfluorosulfonamides were more readily metabolized than other PFAS sulfonates. Biotransformation product prediction, using the chemical transformation simulator, suggested hydrolysis of PFAS sulfonamides to more stable sulfonic acids, which is an important consideration for exposure modeling. This effort greatly expands the PFAS in vitro toxicokinetic dataset, enabling refined TK modeling, in silico tool development, and NAM-based human health evaluations across this important set of emerging contaminants.
Keywords: New Approach Methods (NAMs); PFAS; biotransformation; hepatic clearance; in vitro–in vivo extrapolation (IVIVE); metabolism; toxicokinetics (TK).
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Category-Based Toxicokinetic Evaluations of Data-Poor Per- and Polyfluoroalkyl Substances (PFAS) using Gas Chromatography Coupled with Mass Spectrometry.Toxics. 2023 May 16;11(5):463. doi: 10.3390/toxics11050463. Toxics. 2023. PMID: 37235277 Free PMC article.
-
Targeted Per- and Polyfluoroalkyl substances (PFAS) assessments for high throughput screening: Analytical and testing considerations to inform a PFAS stock quality evaluation framework.Toxicol Appl Pharmacol. 2023 Jan 15;459:116355. doi: 10.1016/j.taap.2022.116355. Epub 2022 Dec 16. Toxicol Appl Pharmacol. 2023. PMID: 36535553 Free PMC article.
-
Review of the zebrafish as a model to investigate per- and polyfluoroalkyl substance toxicity.Toxicol Sci. 2023 Jul 28;194(2):138-152. doi: 10.1093/toxsci/kfad051. Toxicol Sci. 2023. PMID: 37220906 Free PMC article. Review.
-
Potency Ranking of Per- and Polyfluoroalkyl Substances Using High-Throughput Transcriptomic Analysis of Human Liver Spheroids.Toxicol Sci. 2021 Oct 27;184(1):154-169. doi: 10.1093/toxsci/kfab102. Toxicol Sci. 2021. PMID: 34453843
-
A review of the occurrence and microbial transformation of per- and polyfluoroalkyl substances (PFAS) in aqueous film-forming foam (AFFF)-impacted environments.Sci Total Environ. 2024 Jun 1;927:171883. doi: 10.1016/j.scitotenv.2024.171883. Epub 2024 Mar 24. Sci Total Environ. 2024. PMID: 38531439 Review.
Cited by
-
Per- and Polyfluoroalkyl Substances (PFAS) in Consumer Products: An Overview of the Occurrence, Migration, and Exposure Assessment.Molecules. 2025 Feb 21;30(5):994. doi: 10.3390/molecules30050994. Molecules. 2025. PMID: 40076219 Free PMC article. Review.
-
Immunotoxicity of Four Per- and Polyfluoroalkyl Substances Following 28-Day Oral Repeat Dosing in Rats Assessed by the Anti-Sheep Red Blood Cell IgM Response.Toxics. 2025 Jun 10;13(6):490. doi: 10.3390/toxics13060490. Toxics. 2025. PMID: 40559963 Free PMC article.
-
Closing the Gaps in Understanding PFAS Toxicology and Metabolism.Toxics. 2024 Dec 27;13(1):19. doi: 10.3390/toxics13010019. Toxics. 2024. PMID: 39853019 Free PMC article.
-
Non-Targeted Analysis (NTA) of Plasma and Liver from Sprague Dawley Rats Exposed to Perfluorohexanesulfonamide (PFHxSA), a Precursor to Perfluorohexane Sulfonic Acid (PFHxS).Toxics. 2025 Jun 21;13(7):523. doi: 10.3390/toxics13070523. Toxics. 2025. PMID: 40710967 Free PMC article.
References
-
- USEPA Toxic Substances Control Act Reporting and Recordkeeping Requirements for Perfluoroalkyl and Polyfluoroalkyl Substances. Fed. Regist. 2021;88:33926–33966.
-
- Williams A.J., Gaines L.G.T., Grulke C.M., Lowe C.N., Sinclair G.F.B., Samano V., Thillainadarajah I., Meyer B., Patiewicz G., Richard A. Assembly and Curation of Lists of Per- and Polyfluoroalkyl Substances (PFAS) to Support Environmental Science Research. Front. Environ. Sci. 2022;10:1–13. doi: 10.3389/fenvs.2022.850019. - DOI - PMC - PubMed
-
- USEPA . National PFAS Testing Strategy: Identification of Candidate Per- and Polyfluoroalkyl Substances (PFAS) for Testing. USEPA; Washington, DC, USA: 2021.
-
- Houck K.A., Patlewicz G., Richard A.M., Wiulliams A.J., Shobair M.A., Smeltz M., Clifton M.S., Wemore B., Medvedev A., Makarov S. Bioactivity profiling of per- and polyfluoroalkyl substances (PFAS) identifies potential toxicity pathways related to molecular structure. Toxicology. 2021;457:152789. doi: 10.1016/j.tox.2021.152789. - DOI - PubMed
-
- Houck K.A., Paul-Friedman K., Feshuk M., Patlewicz G., Smeltz M., Clifton M.S., Wetmore B.A., Velichko S., Berenyi A., Berg E.L. Evaluation of 147 Perfluoroalkyl Substances for Immunotoxic and Other (patho)Physiological Activities through Phenotypic Screening of Human Primary Cells. volume 40. ALTEX; Terrebonne, QC, Canada: 2023. pp. 248–270. - PMC - PubMed
LinkOut - more resources
Full Text Sources

