Does Catheter Insertion Site Matter? Contamination of Peripheral Intravenous Catheters during Dental Scaling in Dogs
- PMID: 39330786
- PMCID: PMC11435992
- DOI: 10.3390/vetsci11090407
Does Catheter Insertion Site Matter? Contamination of Peripheral Intravenous Catheters during Dental Scaling in Dogs
Abstract
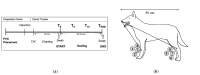
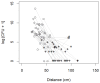
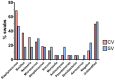
During dental scaling in dogs under general anaesthesia, contamination of the peripheral intravenous catheter (PIVC) is unavoidable due to splatter and the generated aerosol. Bacterial contamination was compared between two commonly used PIVC placement sites. Thirty-nine client-owned dogs with a minimum length from their nose to their tail base of 50 cm were randomly assigned to receive a PIVC in either their cephalic or saphenous vein. Irrespective of the PIVC placement site, brain heart infusion agar dishes were placed in the cephalic and saphenous vein areas. Their lids were closed 0, 5, and 10 min into the procedure. Contamination was measured by counting the colony-forming units after incubation on different substrates. The data were analysed with descriptive statistics, ANOVA, and ANCOVA (p < 0.05). The cephalic vein area showed a significantly higher bacterial load than the saphenous vein area (p ≈ 0.0) regardless of the length of the dog. Furthermore, the dorsal PIVC injection ports were sampled before and after scaling, and the colonies isolated were counted and subjected to MALDI-TOF-MS for identification. The bacteria mainly belonged to the genera Staphylococcus, Neisseria, and Bacillus. Our results suggest that for dental scaling in dogs, the PIVC should be placed in the pelvic limb whenever possible to reduce the potential risk of contamination.
Keywords: cannula; contamination; dental scaling; dog; peripheral intravenous catheter.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Mathews K.A., Brooks M.J., Valliant A.E. A Prospective Study Of Intravenous Catheter Contamination. J. Vet. Emerg. Crit. Care. 1996;6:33–43. doi: 10.1111/j.1476-4431.1996.tb00032.x. - DOI
LinkOut - more resources
Full Text Sources

