Oral Fecal Microbiota Transplantation in Dogs with Tylosin-Responsive Enteropathy-A Proof-of-Concept Study
- PMID: 39330818
- PMCID: PMC11435887
- DOI: 10.3390/vetsci11090439
Oral Fecal Microbiota Transplantation in Dogs with Tylosin-Responsive Enteropathy-A Proof-of-Concept Study
Abstract
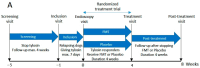
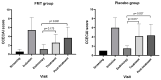
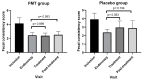
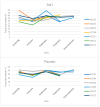
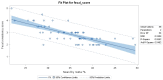
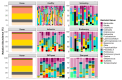
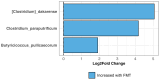
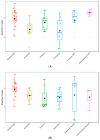
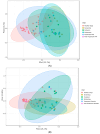
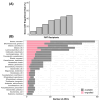
A clinical trial was conducted to evaluate the effect of fecal microbiota transplantation (FMT) on the canine chronic enteropathy clinical activity index (CCECAI), fecal consistency, and microbiome of dogs with tylosin-responsive enteropathy (TRE). The trial consisted of four phases: (1) screening with discontinuation of tylosin for 4 weeks, (2) inclusion with re-introduction of tylosin for 3-7 days, (3) treatment with FMT/placebo for 4 weeks, and (4) post-treatment with follow-up for 4 weeks after treatment cessation. The study found that the treatment efficacy of FMT (71.4%) was slightly higher than that of placebo (50%), but this difference was not statistically significant due to underpowering. The most abundant bacterial species detected in the fecal microbiomes of dogs with TRE before FMT or placebo treatment were Blautia hansenii, Ruminococcus gnavus, Escherichia coli, Clostridium dakarense, Clostridium perfringens, Bacteroides vulgatus, and Faecalimonas umbilicata. After FMT, the microbiomes exhibited increases in Clostridium dakarense, Clostridium paraputrificum, and Butyricicoccus pullicaecorum. The microbiome alpha diversity of TRE dogs was lower when on tylosin treatment compared to healthy dogs, but it increased after treatment in both the FMT and placebo groups. Comparisons with the stool donor showed that, on average, 30.4% of donor strains were engrafted in FMT recipients, with the most common strains being several Blautia sp., Ruminococcus gnavus, unclassified Lachnoclostridium, Collinsella intestinalis, and Fournierella massiliensis.
Keywords: chronic enteropathy; dogs; fecal microbiome; fecal microbiota transplant; microbiome diversity; placebo; tylosin-responsive enteropathy.
Conflict of interest statement
M.H. (Mirja Huhtinen) and T.L. are employed by Orion Corporation, Espoo, Finland, and E.S., C.A.R. and H.G. are employed by AnimalBiome, Oakland, CA, USA.
Figures


Similar articles
-
Effect of a single rectal fecal microbiota transplantation on clinical severity and fecal microbial communities in dogs with chronic inflammatory enteropathy.J Vet Intern Med. 2025 Jan-Feb;39(1):e17264. doi: 10.1111/jvim.17264. J Vet Intern Med. 2025. PMID: 39778887 Free PMC article.
-
Long-term impact of tylosin on fecal microbiota and fecal bile acids of healthy dogs.J Vet Intern Med. 2019 Nov;33(6):2605-2617. doi: 10.1111/jvim.15635. Epub 2019 Oct 31. J Vet Intern Med. 2019. PMID: 31674054 Free PMC article.
-
Machine Learning and Canine Chronic Enteropathies: A New Approach to Investigate FMT Effects.Vet Sci. 2022 Sep 13;9(9):502. doi: 10.3390/vetsci9090502. Vet Sci. 2022. PMID: 36136718 Free PMC article.
-
The Super-Donor Phenomenon in Fecal Microbiota Transplantation.Front Cell Infect Microbiol. 2019 Jan 21;9:2. doi: 10.3389/fcimb.2019.00002. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 30719428 Free PMC article. Review.
-
Fecal transplantation for treatment of inflammatory bowel disease.Cochrane Database Syst Rev. 2018 Nov 13;11(11):CD012774. doi: 10.1002/14651858.CD012774.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2023 Apr 25;4:CD012774. doi: 10.1002/14651858.CD012774.pub3. PMID: 30480772 Free PMC article. Updated.
References
-
- Kilpinen S., Spillmann T., Syrja P., Skrzypczak T., Louhelainen M., Westermarck E. Effect of tylosin on dogs with suspected tylosin-responsive diarrhea: A placebo-controlled, randomized, double-blinded, prospective clinical trial. Acta Vet. Scand. 2011;53:26. doi: 10.1186/1751-0147-53-26. - DOI - PMC - PubMed
-
- Kilpinen S., Spillmann T., Westermarck E. Efficacy of two low-dose oral tylosin regimens in controlling the relapse of diarrhea in dogs with tylosin-responsive diarrhea: A prospective, single-blinded, two-arm parallel, clinical field trial. Acta Vet. Scand. 2014;56:43. doi: 10.1186/s13028-014-0043-5. - DOI - PMC - PubMed
-
- Suchodolski J.S., Dowd S.E., Westermarck E., Steiner J.M., Wolcott R.D., Spillmann T., Harmoinen J.A. The effect of the macrolide antibiotic tylosin on microbial diversity in the canine small intestine as demonstrated by massive parallel 16S rRNA gene sequencing. BMC Microbiol. 2009;9:210. doi: 10.1186/1471-2180-9-210. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

