Apoptosis and Oxidative Stress in Human Intestinal Epithelial Caco-2 Cells Caused by Marine Phycotoxin Azaspiracid-2
- PMID: 39330839
- PMCID: PMC11435587
- DOI: 10.3390/toxins16090381
Apoptosis and Oxidative Stress in Human Intestinal Epithelial Caco-2 Cells Caused by Marine Phycotoxin Azaspiracid-2
Abstract
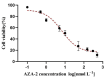
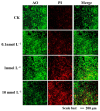
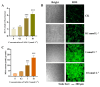
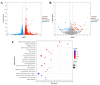
When humans consume seafood contaminated by lipophilic polyether phycotoxins, such as azaspiracids (AZAs), the toxins are mainly leached and absorbed in the small intestine, potentially causing intestinal damage. In this study, human intestinal epithelial Caco-2 cells were used to investigate the adverse effects of azaspiracid-2 (AZA-2) on human intestinal epithelial cells. Cell viability, apoptosis, oxidative damage and mitochondrial ultrastructure were investigated, and ribonucleic acid sequence (RNA-seq) analysis was applied to explore the potential mechanisms of AZA-2 toxicity to Caco-2 cells. Results showed that AZA-2 significantly reduced the proliferation of Caco-2 cells in a concentration-dependent response, and the 48 h EC50 of AZA-2 was 12.65 nmol L-1. AZA-2 can induce apoptosis in Caco-2 cells in a dose-dependent manner. Visible mitochondrial swelling, cristae disintegration, membrane rupture and autophagy were observed in Caco-2 cells exposed to AZA-2. Reactive oxygen species (ROS) and malondialdehyde (MDA) content were significantly increased in Caco-2 cells after 48 h of exposure to 1 and 10 nmol L-1 of AZA-2. Transcriptome analysis showed that KEGG pathways related to cellular oxidative damage and lipid metabolism were affected, mainly including mitophagy, oxidative phosphorylation, cholesterol metabolism, vitamin digestion and absorption, bile secretion and the peroxisome proliferator-activated receptor signaling pathway. The cytotoxic effects of AZA-2 on Caco-2 cells may be associated with ROS-mediated autophagy and apoptosis in mitochondrial cells. Results of this study improve understanding of the cytotoxicity and molecular mechanisms of AZA-2 on Caco-2 cells, which is significant for protecting human health.
Keywords: Caco-2 cells; azaspiracids; cell viability; mitophagy; oxidative damage.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures


Similar articles
-
Absorption and Effect of Azaspiracid-1 Over the Human Intestinal Barrier.Cell Physiol Biochem. 2017;43(1):136-146. doi: 10.1159/000480331. Epub 2017 Aug 28. Cell Physiol Biochem. 2017. PMID: 28848202
-
Coordination regulation of enhanced performance reveals the tolerance mechanism of Chlamys farreri to azaspiracid toxicity.J Hazard Mater. 2024 Sep 5;476:135247. doi: 10.1016/j.jhazmat.2024.135247. Epub 2024 Jul 17. J Hazard Mater. 2024. PMID: 39029196
-
Induction of apoptosis pathways in several cell lines following exposure to the marine algal toxin azaspiracid.Chem Res Toxicol. 2012 Jul 16;25(7):1493-501. doi: 10.1021/tx3001785. Epub 2012 Jul 2. Chem Res Toxicol. 2012. PMID: 22725096
-
Cytotoxicity and intestinal permeability of phycotoxins assessed by the human Caco-2 cell model.Ecotoxicol Environ Saf. 2023 Jan 1;249:114447. doi: 10.1016/j.ecoenv.2022.114447. Epub 2022 Dec 23. Ecotoxicol Environ Saf. 2023. PMID: 38321666 Review.
-
Marine toxins and the cytoskeleton: azaspiracids.FEBS J. 2008 Dec;275(24):6075-81. doi: 10.1111/j.1742-4658.2008.06713.x. Epub 2008 Oct 24. FEBS J. 2008. PMID: 19016861 Review.
Cited by
-
Crosstalk Between Bile Acids and Intestinal Epithelium: Multidimensional Roles of Farnesoid X Receptor and Takeda G Protein Receptor 5.Int J Mol Sci. 2025 Apr 29;26(9):4240. doi: 10.3390/ijms26094240. Int J Mol Sci. 2025. PMID: 40362481 Free PMC article. Review.
-
The Benthic Dinoflagellate Coolia malayensis (Dinophyceae) Produces an Array of Compounds with Antineoplastic Activity in Cells of Tumor Origin.Mar Drugs. 2025 Mar 14;23(3):127. doi: 10.3390/md23030127. Mar Drugs. 2025. PMID: 40137313 Free PMC article.
References
-
- EFSA (European Food Safety Authority) Opinion of the scientific panel on contaminants in the food chain on a request from the European Commission on marine biotoxins in shellfish azaspiracids group. EFSA J. 2008;723:1–52.
-
- Gu H., Luo Z., Krock B., Witt M., Tillmann U. Morphology, phylogeny and azaspiracid profile of Azadinium poporum (Dinophyceae) from the China Sea. Harmful Algae. 2013;21–22:64–75. doi: 10.1016/j.hal.2012.11.009. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

