Suitable Mouse Model to Study Dynamics of West Nile Virus Infection in Culex quinquefasciatus Mosquitoes
- PMID: 39330890
- PMCID: PMC11435581
- DOI: 10.3390/tropicalmed9090201
Suitable Mouse Model to Study Dynamics of West Nile Virus Infection in Culex quinquefasciatus Mosquitoes
Abstract
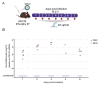
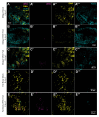
West Nile Virus (WNV) poses a significant global public health threat as a mosquito-borne pathogen. While laboratory mouse models have historically played a crucial role in understanding virus biology, recent research has focused on utilizing immunocompromised models to study arboviruses like dengue and Zika viruses, particularly their interactions with Aedes aegypti mosquitoes. However, there has been a shortage of suitable mouse models for investigating WNV and St. Louis encephalitis virus interactions with their primary vectors, Culex spp. mosquitoes. Here, we establish the AG129 mouse (IFN α/β/γ R-/-) as an effective vertebrate model for examining mosquito-WNV interactions. Following intraperitoneal injection, AG129 mice exhibited transient viremia lasting several days, peaking on the second or third day post-infection, which is sufficient to infect Culex quinquefasciatus mosquitoes during a blood meal. We also observed WNV replication in the midgut and dissemination to other tissues, including the fat body, in infected mosquitoes. Notably, infectious virions were present in the saliva of a viremic AG129 mouse 16 days post-exposure, indicating successful transmission capacity. These findings highlight the utility of AG129 mice for studying vector competence and WNV-mosquito interactions.
Keywords: AG129 mouse; Culex quinquefasciatus; West Nile Virus (WNV); vector competence.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Bidirectional Interactions between Arboviruses and the Bacterial and Viral Microbiota in Aedes aegypti and Culex quinquefasciatus.mBio. 2022 Oct 26;13(5):e0102122. doi: 10.1128/mbio.01021-22. Epub 2022 Sep 7. mBio. 2022. PMID: 36069449 Free PMC article.
-
AG129 Mice as a Comprehensive Model for the Experimental Assessment of Mosquito Vector Competence for Arboviruses.Pathogens. 2022 Aug 3;11(8):879. doi: 10.3390/pathogens11080879. Pathogens. 2022. PMID: 36015000 Free PMC article.
-
Transmission of West Nile virus by Culex quinquefasciatus say infected with Culex Flavivirus Izabal.PLoS Negl Trop Dis. 2010 May 4;4(5):e671. doi: 10.1371/journal.pntd.0000671. PLoS Negl Trop Dis. 2010. PMID: 20454569 Free PMC article.
-
Worldwide transmission and infection risk of mosquito vectors of West Nile, St. Louis encephalitis, Usutu and Japanese encephalitis viruses: a systematic review.Sci Rep. 2023 Jan 6;13(1):308. doi: 10.1038/s41598-022-27236-1. Sci Rep. 2023. PMID: 36609450 Free PMC article.
-
West Nile virus: the Indian scenario.Indian J Med Res. 2003 Sep;118:101-8. Indian J Med Res. 2003. PMID: 14700342 Review.
Cited by
-
Vector Competence of Aedes aegypti from São Tomé and Príncipe for West Nile Virus Transmission.Microorganisms. 2024 Oct 9;12(10):2038. doi: 10.3390/microorganisms12102038. Microorganisms. 2024. PMID: 39458347 Free PMC article.
References
-
- Erazo D., Grant L., Ghisbain G., Marini G., Colón-González F.J., Wint W., Rizzoli A., Van Bortel W., Vogels C.B.F., Grubaugh N.D., et al. Contribution of Climate Change to the Spatial Expansion of West Nile Virus in Europe. Nat. Commun. 2024;15:1196. doi: 10.1038/s41467-024-45290-3. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

