A critical systematic review of extracellular vesicle clinical trials
- PMID: 39330928
- PMCID: PMC11428870
- DOI: 10.1002/jev2.12510
A critical systematic review of extracellular vesicle clinical trials
Abstract
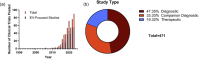
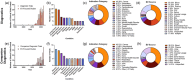
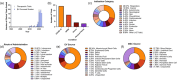
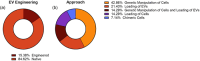
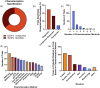
This systematic review examines the landscape of extracellular vesicle (EV)-related clinical trials to elucidate the field's trends in clinical applications and EV-related methodologies, with an additional focus on the acknowledgement of EV subpopulations. By analysing data from public reporting repositories, we catalogued 471 EV-related clinical trials to date, with indications for over 200 diseases. Diagnostics and companion diagnostics represented the bulk of EV-related clinical trials with cancer being the most frequent application. EV-related therapeutics trials mainly utilized mesenchymal stromal cell (MSC) EVs and were most frequently used for treatment of respiratory illnesses. Ultracentrifugation and RNA-sequencing were the most common isolation and characterization techniques; however, methodology for each was not frequently reported in study records. Most of the reported characterization relied on bulk characterization of EV isolates, with only 11% utilizing EV subpopulations in their experimental design. While this may be connected to a lack of available techniques suitable for clinical implementation, it also highlights the opportunity for use of EV subpopulations to improve translational efforts. As academic research identifies more chemically distinct subpopulations and technologies for their enrichment, we forecast to more refined EV trials in the near future. This review emphasizes the need for meticulous methodological reporting and consideration of EV subpopulations to enhance the translational success of EV-based interventions, pointing towards a paradigm shift in personalized medicine.
Keywords: diagnostics; drug delivery vehicles; exosomes; heterogeneity; therapeutics.
© 2024 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


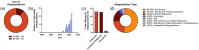
References
-
- Albanese, M. , Chen, Y. A. , Hüls, C. , Gärtner, K. , Tagawa, T. , Mejias‐Perez, E. , Keppler, O. T. , Göbel, C. , Zeidler, R. , Shein, M. , Schütz, A. K. , & Hammerschmidt, W. (2021). MicroRNAs are minor constituents of extracellular vesicles that are rarely delivered to target cells. PLoS Genetics, 17, e1009951. - PMC - PubMed
-
- Bakouny, Z. , Labaki, C. , Bhalla, S. , Schmidt, A. L. , Steinharter, J. A. , Cocco, J. , Tremblay, D. A. , Awad, M. M. , Kessler, A. , Haddad, R. I. , Evans, M. , Busser, F. , Wotman, M. , Curran, C. R. , Zimmerman, B. S. , Bouchard, G. , Jun, T. , Nuzzo, P. V. , Qin, Q. , … Doroshow, D. B. (2022). Oncology clinical trial disruption during the COVID‐19 pandemic: A COVID‐19 and cancer outcomes study. Annals of Oncology: Official Journal of the European Society for Medical Oncology, 33(8), 836–844. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

