Fractional Doses of Pneumococcal Conjugate Vaccine - A Noninferiority Trial
- PMID: 39330966
- PMCID: PMC7616702
- DOI: 10.1056/NEJMoa2314620
Fractional Doses of Pneumococcal Conjugate Vaccine - A Noninferiority Trial
Abstract
Background: Pneumococcal conjugate vaccines are an expensive component of the routine immunization schedule. Fractional-dose regimens may be one option to increase the sustainability of the vaccine program.
Methods: We assessed whether the immunogenicity of fractional doses of the 10-valent and 13-valent pneumococcal conjugate vaccines (PCV10 [GSK] and PCV13 [Pfizer], respectively) would be noninferior to that of the full doses and analyzed the prevalence of vaccine-serotype carriage. We randomly assigned healthy infants in Kenya to one of seven equal-sized trial groups. Participants in groups A through F were assigned to receive either a fractional or full dose of PCV10 or PCV13, administered as two primary doses plus one booster dose. In group A, participants received a full dose of PCV13; group B, a 40% dose of PCV13; group C, a 20% dose of PCV13; group D, a full dose of PCV10; group E, a 40% dose of PCV10; and group F, a 20% dose of PCV10. Participants in the seventh group (group G) received a full dose of PCV10 as three primary doses without a booster. Immunogenicity was assessed 4 weeks after the primary series of doses and 4 weeks after the booster dose. Noninferiority could be declared 4 weeks after the primary series if the difference in the percentage of participants with a threshold response was not more than 10% and 4 weeks after administration of the booster if the ratio of the geometric mean concentration (GMC) of IgG was more than 0.5. A vaccine dose was prespecified as noninferior if it met the noninferiority criterion for at least 8 of the 10 vaccine types in the PCV10 groups or at least 10 of the 13 vaccine types in the PCV13 groups. Carriage was assessed when participants were 9 months and 18 months of age.
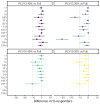
Results: In the per-protocol analysis, 40% of a full dose of PCV13 met the noninferiority criterion for 12 of 13 serotypes after the primary series and for 13 of 13 serotypes after the booster. The immunogenicity of the 20% dose of PCV13 and of the 40% and 20% doses of PCV10 was not noninferior to that of the full doses. The prevalence of vaccine-serotype carriage was similar across the PCV13 groups at 9 months and 18 months of age.
Conclusions: In a three-dose schedule (two primary doses and a booster), 40% doses of PCV13 were noninferior to full doses for all included serotypes. Lower doses of PCV13 and PCV10 did not meet the criteria for noninferiority. (Funded by the Bill and Melinda Gates Foundation and others; ClinicalTrials.gov number, NCT03489018; Pan African Clinical Trial Registry number, PACTR202104717648755.).
Copyright © 2024 Massachusetts Medical Society.
Figures

References
-
- Hammitt LL, Etyang AO, Morpeth SC, et al. Effect of ten-valent pneumococcal conjugate vaccine on invasive pneumococcal disease and nasopharyngeal carriage in Kenya: a longitudinal surveillance study. Lancet (London, England) 2019;393(10186):2146–2154. doi: 10.1016/S0140-6736(18)33005-8. In eng. - DOI - PMC - PubMed
-
- Reyburn R, Tsatsaronis A, von Mollendorf C, Mulholland K, Russell FM. Systematic review on the impact of the pneumococcal conjugate vaccine ten valent (PCV10) or thirteen valent (PCV13) on all-cause, radiologically confirmed and severe pneumonia hospitalisation rates and pneumonia mortality in children 0-9 years old. J Glob Health. 2023;13:05002. doi: 10.7189/jogh.13.05002. In eng. - DOI - PMC - PubMed
-
- Gavi. Gavi’s pneumococcal support. http://www.gavi.org/support/nvs/pneumococcal/
-
- World Health Organization. Pneumococcal vaccines WHO position paper--2019. Releve epidemiologique hebdomadaire / Section d’hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record / Health Section of the Secretariat of the League of Nations. 2019;8(94):85–104. In eng fre.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
