Use of the senolytics dasatinib and quercetin for prevention of pelvic organ prolapse in a mouse animal model
- PMID: 39331015
- PMCID: PMC11501376
- DOI: 10.18632/aging.206120
Use of the senolytics dasatinib and quercetin for prevention of pelvic organ prolapse in a mouse animal model
Abstract
Objective: Senolytic agents have the potential to target age-related pathology associated with cellular senescence and reduce senescent cell activity in several disease processes. We utilized a mouse model of pelvic organ prolapse, Fibulin-5 knockout (Fbln-5-/-) mice, to assess the ability of dasatinib and quercetin (D+Q) to prevent development of prolapse.
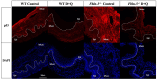
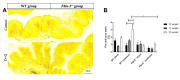
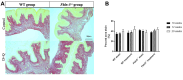
Methods: Four-week-old female Fbln-5-/- (n=63) and wild-type (WT) mice (n=54) were assigned to control (vehicle injection) or treatment (D = 5 mg/kg, Q = 50 mg/kg) groups. Weekly oral gavage injections were administered from weeks 4-8 of life. Pelvic organ prolapse quantification system measurements were obtained weekly. Vaginal tissue was harvested at 10, 12 and 20 weeks. Tissue analysis included immunostaining for cell cycle inhibitors, multiplex cytokine analysis, senescence-associated-β-galactosidase (SA-β-Gal) and histologic analysis of extracellular matrix proteins.
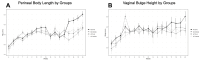
Results: Perineal body length was significantly longer in Fbln-5-/- treatment mice at 20 weeks. Expression of p16 and p53 was decreased in Fbln-5-/- treatment mice compared to controls (4.0% vs. 26.7%, p=0.0124 and 2.9% vs. 16.8%, p=0.272) at 20 weeks. Expression of SA-β-Gal and senescence-associated cytokines did not vary significantly between groups. At 20 weeks, vaginal tissue elastin content in Fbln-5-/- treatment mice increased compared to controls (1.04% vs. 0.84%, p=0.999).
Conclusions: D+Q injections did not result in clinically significant differences in prolapse development but did demonstrate decreased expression of cellular senescence markers in Fbln-5-/- mice. This suggests senolytic agents may mitigate contributions of cellular senescence to tissue dysfunction associated with prolapse. Further studies are needed to confirm ideal timing, dosage, and route of senolytics in prevention of prolapse.
Keywords: animal model; cellular senescence; pelvic organ prolapse; senolytic agents.
Conflict of interest statement
Figures

References
-
- American College of Obstetricians and Gynecologists and the American Urogynecologic Society, and INTERIM UPDATE: This Practice Bulletin is updated as highlighted to reflect the US Food and Drug Administration order to stop the sale of transvaginal synthetic mesh products for the repair of pelvic organ prolapse. Pelvic Organ Prolapse. Female Pelvic Med Reconstr Surg. 2019; 25:397–408. 10.1097/SPV.0000000000000794 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

