Biofluid Markers and Tissue Biopsies Analyses for the Prodromal and Earliest Phase of Parkinson's Disease
- PMID: 39331105
- PMCID: PMC11494635
- DOI: 10.3233/JPD-240007
Biofluid Markers and Tissue Biopsies Analyses for the Prodromal and Earliest Phase of Parkinson's Disease
Abstract
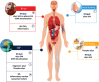
The recent development of new methods to detect misfolded α-synuclein (αSyn) aggregates in biofluids and tissue biopsies in the earliest Parkinson's disease (PD) phases is dramatically challenging the biological definition of PD. The αSyn seed amplification methods in cerebrospinal fluid (CSF) showed high sensitivity and specificity for early diagnosis of PD and Lewy bodies disorders. Several studies in isolated REM sleep behavior disorders and other at-risk populations also demonstrated a high prevalence of CSF αSyn positivity and its potential value in predicting the phenoconversion to clinically manifested diseases. Growing evidence exists for αSyn aggregates in olfactory mucosa, skin, and other tissues in subjects with PD or at-risk subjects. DOPA decarboxylase and numerous other candidates have been additionally proposed for either diagnostic or prognostic purposes in earliest PD phases. The newly described αSyn detection in blood, through its quantification in neuronally-derived exosome vesicles, represents a technical challenge that could open a new scenario for the biological diagnosis of PD. Despite this growing evidence in the field, most of method of αSyn detection and markers still need to be validated in ongoing longitudinal studies through an accurate assessment of different prodromal disease subtypes and scenarios before being definitively implemented in clinical settings.
Keywords: DOPA decarboxylase; cerebrospinal fluid; plasma biomarkers; prodromal Parkinson’s disease; seed amplification assays; α-synuclein.
Conflict of interest statement
The authors have no conflict of interest to report.
Nobutaka Hattori is part of the Editorial Board Member of this journal but was not involved in the peer-review process of this article nor had access to any information regarding its peer-review
Figures
References
-
- Cardoso F, Goetz CG, Mestre TA, et al. A statement of the MDS on biological definition, staging, and classification of Parkinson’s disease. Mov Disord. 2024; 39: 259–266. - PubMed
-
- Höglinger GU, Adler CH, Berg D, et al. Towards a biological definition of Parkinson’s disease. Preprints. 2023; 10.20944/preprints202304.0108.v1 [Preprint]. Posted April 7, 2023. - DOI
-
- Heinzel S, Berg D, Gasser T, et al. Update of the MDS research criteria for prodromal Parkinson’s disease. Mov Disord. 2019; 34: 1464–1470. - PubMed
-
- Pilotto A, Heinzel S, Suenkel U, et al. Application of the movement disorder society prodromal Parkinson’s disease research criteria in 2 independent prospective cohorts. Mov Disord. 2017; 32: 1025–1034. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

