Selecting the Best Pharmacokinetic Models for a Priori Model-Informed Precision Dosing with Model Ensembling
- PMID: 39331236
- PMCID: PMC11522197
- DOI: 10.1007/s40262-024-01425-9
Selecting the Best Pharmacokinetic Models for a Priori Model-Informed Precision Dosing with Model Ensembling
Abstract
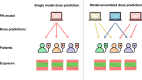
Background and objective: When utilizing population pharmacokinetic (popPK) models for a priori dosage individualization, selecting the best model is crucial to obtain adequate doses. We developed and evaluated several model-selection and ensembling methods, using external evaluation on the basis of therapeutic drug monitoring (TDM) samples to identify the best (set of) models per patient for a priori dosage individualization.
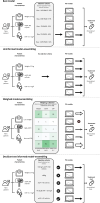
Methods: PK data and models describing both hospitalized patients (n = 134) receiving continuous vancomycin (26 models) and patients (n = 92) receiving imatinib in an outpatient setting (12 models) are included. Target attainment of four model-selection methods was compared with standard dosing: the best model based on external validation, uninformed model ensembling, model ensembling using a weighting scheme on the basis of covariate-stratified external evaluation, and model selection using covariates in decision trees that were subsequently ensembled.
Results: Overall, the use of PK models improved the proportion of patients exposed to concentrations within the therapeutic window for both cohorts. Relative improvement of proportion on target for best model, unweighted, weighted, and decision trees were - 7.0%, 2.3%, 11.4%, and 37.0% (vancomycin method-development); 23.2%, 7.9%, 15.6%, and, 77.2% (vancomycin validation); 40.7%, 50.0%, 59.5%, and 59.5% (imatinib method-development); and 19.0%, 28.5%, 38.0%, and 23.8% (imatinib validation), respectively.
Conclusions: The best (set of) models per patient for a priori dosage individualization can be identified using a relatively small set of TDM samples as external evaluation. Adequately performing popPK models were identified while also excluding poor-performing models. Dose recommendations resulted in more patients within the therapeutic range for both vancomycin and imatinib. Prospective validation is necessary before clinical implementation.
© 2024. The Author(s).
Conflict of interest statement
The authors declared no competing interests for this work.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

