Sacituzumab Govitecan for Second and Subsequent Line Palliative Treatment of Patients with Triple-Negative Breast Cancer: A Polish Real-World Multicenter Cohort Study
- PMID: 39331319
- PMCID: PMC11573939
- DOI: 10.1007/s40487-024-00307-1
Sacituzumab Govitecan for Second and Subsequent Line Palliative Treatment of Patients with Triple-Negative Breast Cancer: A Polish Real-World Multicenter Cohort Study
Abstract
Introduction: Sacituzumab govitecan (SG) is approved for patients with previously treated metastatic or locally advanced triple-negative breast cancer (TNBC), as per the ASCENT trial results. Real-world studies (RWSs) cover more diverse patients than clinical trials, offering crucial data for healthcare policies. This study aimed to investigate the safety and efficacy of SG in real-world Polish patients with previously treated metastatic TNBC.
Methods: In this ambispective multicenter cohort study, we collected demographic and clinical data. Premedication, adjustments in SG dosage, and treatment regimen adhered to the product's characteristics.
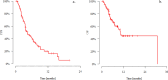
Results: We included 79 female patients. The median age at SG initiation was 53 years; 32% of patients were initially diagnosed with a non-TNBC subtype. The median number of previous palliative lines was 2. Seven patients presented with brain metastases. The median overall survival was 10.3 months, and the median progression-free survival (PFS) was 4.4 months. The overall response rate was 35%, with a median time to response of 2 months. SG was discontinued by 70% of patients, primarily due to disease progression (95%). Treatment delays due to adverse events (AEs) occurred in 67% and dose reductions in 25% of patients, with neutropenia being the most common. Grade ≥ 2 AEs included neutropenia (43%), anemia (10.1%), and diarrhea (4%). A longer interval between breast cancer diagnosis and SG initiation or between metastasis diagnosis and SG initiation correlated with improved PFS, likely reflecting the disease's biological aggressiveness rather than treatment efficacy.
Conclusion: In this RWS, SG demonstrated effectiveness and safety in patients with previously treated metastatic TNBC, consistent with ASCENT trial outcomes. Further research is needed to explore the efficacy of SG in different patient populations and healthcare systems.
Keywords: Metastases; Poland; Real-world study; Sacituzumab govitecan; Triple-negative breast cancer.
© 2024. The Author(s).
Conflict of interest statement
Figures
References
-
- Yersal O, Barutca S. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J Clin Oncol. 2014;5(3):412–24. Available from: https://pubmed.ncbi.nlm.nih.gov/25114856/. Accessed 29 Mar 2024. - PMC - PubMed
-
- Triple-negative Breast Cancer | ESMO https://www.esmo.org/living-guidelines/esmo-metastatic-breast-cancer-living-guideline/triple-negative-breast-cancer. Available from: https://www.esmo.org/living-guidelines/esmo-metastatic-breast-cancer-liv.... Accessed 28 May 2024
-
- Rashmi Kumar N, Schonfeld R, Gradishar WJ, Lurie RH, Moran MS, Abraham J, et al. NCCN Guidelines Version 2.2024 Breast Cancer. 2024. Available from: https://www.nccn.org. Accessed 3 Apr 2024.
-
- Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396(10265):1817–28. Available from: https://pubmed.ncbi.nlm.nih.gov/33278935/. Accessed 23 Mar 2023. - PubMed
LinkOut - more resources
Full Text Sources