An in vivo tumour organoid model based on the chick embryonic chorioallantoic membrane mimics key characteristics of the patient tissue: a proof-of-concept study
- PMID: 39331331
- PMCID: PMC11436503
- DOI: 10.1186/s13550-024-01151-0
An in vivo tumour organoid model based on the chick embryonic chorioallantoic membrane mimics key characteristics of the patient tissue: a proof-of-concept study
Abstract
Background: Patient-derived tumour organoids (PDOs) are highly advanced in vitro models for disease modelling, yet they lack vascularisation. To overcome this shortcoming, organoids can be inoculated onto the chorioallantoic membrane (CAM); the highly vascularised, not innervated extraembryonic membrane of fertilised chicken eggs. Therefore, we aimed to (1) establish a CAM patient-derived xenograft (PDX) model based on PDOs generated from the liver metastasis of a colorectal cancer (CRC) patient and (2) to evaluate the translational pipeline (patient - in vitro PDOs - in vivo CAM-PDX) regarding morphology, histopathology, expression of C-X-C chemokine receptor type 4 (CXCR4), and radiotracer uptake patterns.
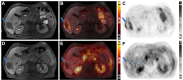
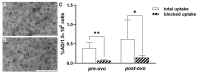
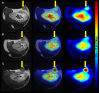
Results: The main liver metastasis of the CRC patient exhibited high 2-[18F]FDG uptake and moderate and focal [68Ga]Ga-Pentixafor accumulation in the peripheral part of the metastasis. Inoculation of PDOs derived from this region onto the CAM resulted in large, highly viable, and extensively vascularised xenografts, as demonstrated immunohistochemically and confirmed by high 2-[18F]FDG uptake. The xenografts showed striking histomorphological similarity to the patient's liver metastasis. The moderate expression of CXCR4 was maintained in ovo and was concordant with the expression levels of the patient's sample and in vitro PDOs. Following in vitro re-culturing of CAM-PDXs, growth, and [68Ga]Ga-Pentixafor uptake were unaltered compared to PDOs before transplantation onto the CAM. Although [68Ga]Ga-Pentixafor was taken up into CAM-PDXs, the uptake in the baseline and blocking group were comparable and there was only a trend towards blocking.
Conclusions: We successfully established an in vivo CAM-PDX model based on CRC PDOs. The histomorphological features and target protein expression of the original patient's tissue were mirrored in the in vitro PDOs, and particularly in the in vivo CAM-PDXs. The [68Ga]Ga-Pentixafor uptake patterns were comparable between in vitro, in ovo and clinical data and 2-[18F]FDG was avidly taken up in the patient's liver metastasis and CAM-PDXs. We thus propose the CAM-PDX model as an alternative in vivo model with promising translational value for CRC patients.
Keywords: 2-[18F]FDG; CAM; CRC; In ovo; PDX; PET/MRI; Patient-derived organoids; [68Ga]Ga-Pentixafor.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. 10.3322/caac.21660. - PubMed
-
- Kim J, Takeuchi H, Lam ST, Turner RR, Wang H-J, Kuo C, et al. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23:2744–53. 10.1200/JCO.2005.07.078. - PubMed
-
- Schimanski CC, Schwald S, Simiantonaki N, Jayasinghe C, Gönner U, Wilsberg V, et al. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res. 2005;11:1743–50. 10.1158/1078-0432.CCR-04-1195. - PubMed
-
- Sato T, Vries RG, Snippert HJ, Van De Wetering M, Barker N, Stange DE, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–5. 10.1038/nature07935. - PubMed
LinkOut - more resources
Full Text Sources

