Pharmacologically Targeting Ferroptosis and Cuproptosis in Neuroblastoma
- PMID: 39331355
- PMCID: PMC11790790
- DOI: 10.1007/s12035-024-04501-0
Pharmacologically Targeting Ferroptosis and Cuproptosis in Neuroblastoma
Abstract
Neuroblastoma is a deadly pediatric cancer that originates from the neural crest and frequently develops in the abdomen or adrenal gland. Although multiple approaches, including chemotherapy, radiotherapy, targeted therapy, and immunotherapy, are recommended for treating neuroblastoma, the tumor will eventually develop resistance, leading to treatment failure and cancer relapse. Therefore, a firm understanding of the molecular mechanisms underlying therapeutic resistance is vital for the development of new effective therapies. Recent research suggests that cancer-specific modifications to multiple subtypes of nonapoptotic regulated cell death (RCD), such as ferroptosis and cuproptosis, contribute to therapeutic resistance in neuroblastoma. Targeting these specific types of RCD may be viable novel targets for future drug discovery in the treatment of neuroblastoma. In this review, we summarize the core mechanisms by which the inability to properly execute ferroptosis and cuproptosis can enhance the pathogenesis of neuroblastoma. Therefore, we focus on emerging therapeutic compounds that can induce ferroptosis or cuproptosis, delineating their beneficial pharmacodynamic effects in neuroblastoma treatment. Cumulatively, we suggest that the pharmacological stimulation of ferroptosis and ferroptosis may be a novel and therapeutically viable strategy to target neuroblastoma.
Keywords: Ferroptosis; Compounds; Cuproptosis; Neuroblastoma.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics Approval and Consent to Participate: Not applicable. Consent for Publication: Not applicable. Competing Interests: The authors declare no competing interests.
Figures
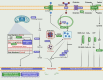
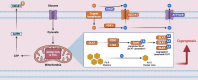
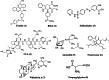
References
Publication types
MeSH terms
Substances
Grants and funding
- 2022-MS-209/Natural Science Foundation of Liaoning Province
- 2023JH2/20200119/2023 China Medical University High-quality Development Fund
- YJZX202207/Henan Pediatric Disease Clinical Medical Research Center Foundation
- CX-B-2021-02/CAAE Epilepsy Research Fund
- 2022-N004-09/Medical Education Research Project of Liaoning Province
LinkOut - more resources
Full Text Sources
Medical

