Treatment Effects in Randomized and Nonrandomized Studies of Pharmacological Interventions: A Meta-Analysis
- PMID: 39331390
- PMCID: PMC11437387
- DOI: 10.1001/jamanetworkopen.2024.36230
Treatment Effects in Randomized and Nonrandomized Studies of Pharmacological Interventions: A Meta-Analysis
Abstract
Importance: Randomized clinical trials (RCTs) are widely regarded as the methodological benchmark for assessing clinical efficacy and safety of health interventions. There is growing interest in using nonrandomized studies to assess efficacy and safety of new drugs.
Objective: To determine how treatment effects for the same drug compare when evaluated in nonrandomized vs randomized studies.
Data sources: Meta-analyses published between 2009 and 2018 were identified in MEDLINE via PubMed and the Cochrane Database of Systematic Reviews. Data analysis was conducted from October 2019 to July 2024.
Study selection: Meta-analyses of pharmacological interventions were eligible for inclusion if both randomized and nonrandomized studies contributed to a single meta-analytic estimate.
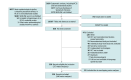
Data extraction and synthesis: For this meta-analysis using a meta-epidemiological framework, separate summary effect size estimates were calculated for nonrandomized and randomized studies within each meta-analysis using a random-effects model and then these estimates were compared. The reporting of this study followed the Guidelines for Reporting Meta-Epidemiological Methodology Research and relevant portions of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline.
Main outcome and measures: The primary outcome was discrepancies in treatment effects obtained from nonrandomized and randomized studies, as measured by the proportion of meta-analyses where the 2 study types disagreed about the direction or magnitude of effect, disagreed beyond chance about the effect size estimate, and the summary ratio of odds ratios (ROR) obtained from nonrandomized vs randomized studies combined across all meta-analyses.
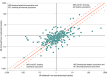
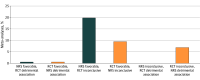
Results: A total of 346 meta-analyses with 2746 studies were included. Statistical conclusions about drug benefits and harms were different for 130 of 346 meta-analyses (37.6%) when focusing solely on either nonrandomized or randomized studies. Disagreements were beyond chance for 54 meta-analyses (15.6%). Across all meta-analyses, there was no strong evidence of consistent differences in treatment effects obtained from nonrandomized vs randomized studies (summary ROR, 0.95; 95% credible interval [CrI], 0.89-1.02). Compared with experimental nonrandomized studies, randomized studies produced on average a 19% smaller treatment effect (ROR, 0.81; 95% CrI, 0.68-0.97). There was increased heterogeneity in effect size estimates obtained from nonrandomized compared with randomized studies.
Conclusions and relevance: In this meta-analysis of treatment effects of pharmacological interventions obtained from randomized and nonrandomized studies, there was no overall difference in effect size estimates between study types on average, but nonrandomized studies both overestimated and underestimated treatment effects observed in randomized studies and introduced additional uncertainty. These findings suggest that relying on nonrandomized studies as substitutes for RCTs may introduce additional uncertainty about the therapeutic effects of new drugs.
Conflict of interest statement
Figures

References
-
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use . ICH harmonised guideline: general considerations for clinical studies E8(R1). October 6, 2021. Accessed September 3, 2023. https://database.ich.org/sites/default/files/E8-R1_Guideline_Step4_2021_...
-
- European Medicines Agency . Real-world evidence framework to support EU regulatory decision-making: report on the experience gained with regulator-led studies from September 2021 to February 2023. Amsterdam: European Medicines Agency. 2023. Accessed September 15, 2023. https://www.ema.europa.eu/en/documents/report/real-world-evidence-framew...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

