Human induced pluripotent stem cell-derived cardiomyocytes to study inflammation-induced aberrant calcium transient
- PMID: 39331464
- PMCID: PMC11434618
- DOI: 10.7554/eLife.95867
Human induced pluripotent stem cell-derived cardiomyocytes to study inflammation-induced aberrant calcium transient
Abstract
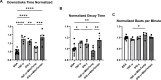
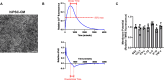
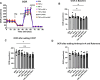
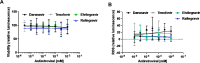
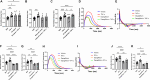
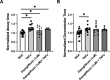
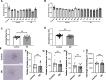
Heart failure with preserved ejection fraction (HFpEF) is commonly found in persons living with HIV (PLWH) even when antiretroviral therapy suppresses HIV viremia. However, studying this condition has been challenging because an appropriate animal model is not available. In this article, we studied calcium transient in human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) in culture to simulate the cardiomyocyte relaxation defect noted in PLWH and HFpEF and assess whether various drugs have an effect. We show that treatment of hiPSC-CMs with inflammatory cytokines (such as interferon-γ or TNF-α) impairs their Ca2+ uptake into sarcoplasmic reticulum and that SGLT2 inhibitors, clinically proven as effective for HFpEF, reverse this effect. Additionally, treatment with mitochondrial antioxidants (like mito-Tempo) and certain antiretrovirals resulted in the reversal of the effects of these cytokines on calcium transient. Finally, incubation of hiPSC-CMs with serum from HIV patients with and without diastolic dysfunction did not alter their Ca2+-decay time, indicating that the exposure to the serum of these patients is not sufficient to induce the decrease in Ca2+ uptake in vitro. Together, our results indicate that hiPSC-CMs can be used as a model to study molecular mechanisms of inflammation-mediated abnormal cardiomyocyte relaxation and screen for potential new interventions.
Keywords: calcium transient; heart failure with preserved ejection fraction; human; human immunodeficiency virus; human induced pluripotent stem cell-derived cardiomyocyte; infectious disease; inflammation; microbiology.
© 2024, Tatekoshi, Chen et al.
Conflict of interest statement
YT, CC, JS, HC, MB, DL, PB, MF, RD, PH No competing interests declared, HA Reviewing editor, eLife
Figures
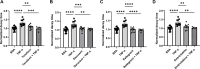



Update of
- doi: 10.1101/2024.01.17.575991
- doi: 10.7554/eLife.95867.1
- doi: 10.7554/eLife.95867.2
References
-
- Amirayan-Chevillard N, Tissot-Dupont H, Capo C, Brunet C, Dignat-George F, Obadia Y, Gallais H, Mege JL. Impact of highly active anti-retroviral therapy (HAART) on cytokine production and monocyte subsets in HIV-infected patients. Clinical and Experimental Immunology. 2000;120:107–112. doi: 10.1046/j.1365-2249.2000.01201.x. - DOI - PMC - PubMed
-
- Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca H-P, Choi D-J, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M, EMPEROR-Preserved Trial Investigators Empagliflozin in heart failure with a preserved ejection fraction. The New England Journal of Medicine. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038. - DOI - PubMed
-
- Arnold JR, Kanagala P, Budgeon CA, Jerosch-Herold M, Gulsin GS, Singh A, Khan JN, Chan DCS, Squire IB, Ng LL, McCann GP. Prevalence and prognostic significance of microvascular dysfunction in heart failure with preserved ejection fraction. JACC. Cardiovascular Imaging. 2022;15:1001–1011. doi: 10.1016/j.jcmg.2021.11.022. - DOI - PubMed
-
- Bahrami H, Budoff M, Haberlen SA, Rezaeian P, Ketlogetswe K, Tracy R, Palella F, Witt MD, McConnell MV, Kingsley L, Post WS. Inflammatory markers associated with subclinical coronary artery disease: The multicenter AIDS cohort study. Journal of the American Heart Association. 2016;5:e003371. doi: 10.1161/JAHA.116.003371. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

