Sleep induced by mechanosensory stimulation provides cognitive and health benefits in Drosophila
- PMID: 39331490
- PMCID: PMC12104162
- DOI: 10.1093/sleep/zsae226
Sleep induced by mechanosensory stimulation provides cognitive and health benefits in Drosophila
Abstract
Study objectives: Sleep is a complex phenomenon regulated by various factors, including sensory input. Anecdotal observations have suggested that gentle rocking helps babies fall asleep, and experimental studies have verified that rocking promotes sleep in both humans and mice. Recent studies have expanded this understanding, demonstrating that gentle vibration also induces sleep in Drosophila. Natural sleep serves multiple functions, including learning and memory, synaptic downscaling, and reduction of harmful substances associated with neurodegenerative diseases. Here, we investigated whether vibration-induced sleep (VIS) provides similar cognitive and health benefits in Drosophila.
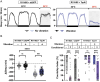
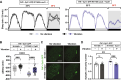
Methods: We administered gentle vibration to flies that slept very little due to a forced activation of wake-promoting neurons and investigated how the vibration influenced learning and memory in the courtship conditioning paradigm. Additionally, we examined the effects of VIS on synaptic downscaling by counting synaptic varicosities of select neurons. Finally, we determined whether vibration could induce sleep in Drosophila models of Alzheimer's disease (AD) and suppress the accumulation of Amyloid β (Aβ) and Tubulin Associated Unit (TAU).
Results: VIS enhanced performance in a courtship conditioning paradigm and reduced the number of synaptic varicosities in select neurons. Moreover, vibration improved sleep in Drosophila models of AD, reducing Aβ and TAU levels.
Conclusions: Mechanosensory stimulation offers a promising noninvasive avenue for enhancing sleep, potentially providing associated cognitive and health benefits.
Keywords: Drosophila; Alzheimer’s disease; Amyloid-β; TAU; learning; memory; sleep; synapse; vibration.
© The Author(s) 2024. Published by Oxford University Press on behalf of Sleep Research Society. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Figures



Update of
-
Sleep induced by mechanosensory stimulation provides cognitive and health benefits in Drosophila.bioRxiv [Preprint]. 2024 Jul 12:2024.07.10.602891. doi: 10.1101/2024.07.10.602891. bioRxiv. 2024. Update in: Sleep. 2024 Dec 11;47(12):zsae226. doi: 10.1093/sleep/zsae226. PMID: 39026689 Free PMC article. Updated. Preprint.
Comment in
-
The power of the rocking cradle: improving sleep function by gentle vibration.Sleep. 2024 Dec 11;47(12):zsae245. doi: 10.1093/sleep/zsae245. Sleep. 2024. PMID: 39441991 No abstract available.
References
-
- Duhart JM, Inami S, Koh K. Many faces of sleep regulation: beyond the time of day and prior wake time. FEBS J. 2021;290:931–950. doi: https://doi.org/10.1111/febs.16320 - DOI - PMC - PubMed
-
- Bayer L, Constantinescu I, Perrig S, et al. Rocking synchronizes brain waves during a short nap. Curr Biol. 2011;21(12):R461–R462. doi: https://doi.org/10.1016/j.cub.2011.05.012 - DOI - PubMed
-
- Perrault AA, Khani A, Quairiaux C, et al. Whole-night continuous rocking entrains spontaneous neural oscillations with benefits for sleep and memory. Curr Biol. 2019;29:402–411.e3. doi: https://doi.org/10.1016/j.cub.2018.12.028 - DOI - PubMed
-
- Kompotis K, Hubbard J, Emmenegger Y, et al. Rocking promotes sleep in mice through rhythmic stimulation of the vestibular system. Curr Biol. 2019;29:392–401.e4. doi: https://doi.org/10.1016/j.cub.2018.12.007 - DOI - PubMed
-
- van Sluijs RM, Rondei QJ, Schluep D, et al. Effect of rocking movements on afternoon sleep. Front Neurosci. 2020;13(January):1446. doi: https://doi.org/10.3389/fnins.2019.01446 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

