The effect of allergen immunotherapy in patients with central compartment atopic disease post-surgery
- PMID: 39331586
- PMCID: PMC11785156
- DOI: 10.1002/alr.23459
The effect of allergen immunotherapy in patients with central compartment atopic disease post-surgery
Abstract
Objective: To assess the effect of allergen immunotherapy (AIT) on patients with central compartment atopic disease (CCAD) and house dust mite (HDM) sensitization post-surgery.
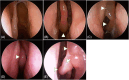
Methods: A retrospective cohort of surgically treated, HDM-sensitized CRSwNP patients phenotyped as CCAD was assessed. Patients were divided into two groups based on whether they had AIT commenced as part of their surgical care. All AIT patients started immunotherapy prior to their surgery. The primary endpoint was reformation of middle turbinate (MT) edema 12 months postsurgery. Secondary endpoints were corticosteroid irrigation use (<4 times/week vs. ≥4 times/week, %) and the rhinologic domain of the 22-item sino-nasal outcome test (SNOT-22). Demographic characteristics, concomitant asthma, smoking status, history of aspirin-exacerbated respiratory disease, conjunctival symptoms, polysensitization, serum eosinophils (cell × 109/L), tissue eosinophilia (% > 100/HPF), and serum IgE (kU/L) were also recorded.
Results: Eighty-six CCAD patients were assessed (41 ± 14 yrs, 64% female). AIT was applied in 37% (n = 32). Baseline features were similar apart from greater conjunctival symptoms (72 vs. 45%, p = 0.02) in the AIT group. At 12 months post-surgery, the AIT group has less MT edema (% ≥ diffuse 15.6 vs. 52.9, p < 0.01). Patients on AIT also had less pharmacotherapy requirements at 12 months (% ≥ 4/week, 37.5 vs. 79.6%, p < 0.01). The rhinologic symptoms were similar (21.1 ± 17.1 vs. 20.1 ± 21.6, p = 0.83).
Conclusions: Surgery and pharmacotherapy are effective in managing CCAD, but the addition of AIT improved allergic phenomenon and allowed de-escalation of topical therapy. Longer term studies are required to demonstrate further immunomodulation.
Keywords: desensitization; hypersensitivity; immunologic; nasal surgical procedures; sinusitis.
© 2024 The Author(s). International Forum of Allergy & Rhinology published by Wiley Periodicals LLC on behalf of American Academy of Otolaryngic Allergy and American Rhinologic Society.
Conflict of interest statement
Richard J. Harvey is consultant/advisory board with Medtronic, Novartis, Glaxo‐Smith‐Kline, and Meda Pharmaceuticals. He has been on the speakers’ bureau for Glaxo‐Smith‐Kline, Astra‐Zeneca, Meda Pharmaceuticals, and Seqirus. Larry Kalish is on the speakers’ bureau for Viatris, Stallergenens, and Seqirus Pharmaceuticals. Raewyn G. Campbell is on the speaker's bureau for Medtronic, Viatris, and Glaxo‐Smith‐Kline. All other authors have no personal, financial, or institutional interest in any drugs, materials, or devices described in this article.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

