Long-term outcomes in patients with advanced intrahepatic cholangiocarcinoma treated with hepatic arterial infusion chemotherapy
- PMID: 39331613
- PMCID: PMC11807433
- DOI: 10.1093/jnci/djae202
Long-term outcomes in patients with advanced intrahepatic cholangiocarcinoma treated with hepatic arterial infusion chemotherapy
Abstract
Background: Hepatic artery infusion of chemotherapy has demonstrated disease control and suggested improvement in overall survival in intrahepatic cholangiocarcinoma. We report herein the long-term results and role of molecular alterations of a phase II clinical trial of hepatic artery infusion chemotherapy plus systemic chemotherapy, with a retrospective cohort of patients treated with hepatic artery infusion at Memorial Sloan Kettering Cancer Center.
Methods: This is a secondary analysis of a single-institution, phase II trial, and retrospective cohort of unresectable intrahepatic cholangiocarcinoma treated with hepatic artery infusion floxuridine plus systemic gemcitabine and oxaliplatin. The primary aim was to assess long-term oncologic outcomes. A subset underwent tissue-based genomic sequencing, and molecular alterations were correlated with progression-free survival (PFS) and overall survival.
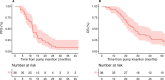
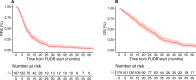
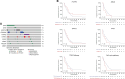
Results: A total of 38 patients were treated on trial with a median follow-up of 76.9 months. Median PFS was 11.8 months (95% confidence interval [CI] = 11 to 15.1 months). The median overall survival was 26.8 months (95% CI = 20.9 to 40.6 months). The 1-, 2-, and 5-year overall survival rate was 89.5%, 55%, and 21%, respectively. Nine (24%) patients received hepatic artery infusion with mitomycin C post-floxuridine progression with an objective response rate of 44% and a median PFS of 3.93 months (95% CI = 2.33 months to not reached). A total of 170 patients not treated on the clinical trial were included in a retrospective analysis. Median PFS and overall survival were 7.93 months (95% CI = 7.27 to 10.07 months) and 22.5 months (95% CI = 19.5 to 28.3 months), respectively. Alterations in the TP53 and cell-cycle pathway had a worse PFS to hepatic artery infusion-based therapy compared with wild-type disease.
Conclusion: In locally advanced intrahepatic cholangiocarcinoma, hepatic artery infusion with floxuridine in combination with systemic therapy can offer long-term durable disease control. Molecular alterations may predict for response.
© The Author(s) 2024. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
Mithat Gönen has had paid consulting or advisory roles for Tesaro. James J. Harding reports personal fees from Adaptimmune, Bristol Myers Squibb, CytomX, Eli Lilly, Exelixis, Hepion, MedVir, Tempus, QED, Zymeworks and research support from Bristol Myers Squibb, Boehringer Ingelheim, Calithera, CytomX, Eli Lilly, Genoscience, Loxo, Novartis, Pfizer, Polaris, Yiviva, and Zymeworks. Diane Reidy-Lagunes reports research support from Merck, Ipsen, and Novartis and consulting fees from AAA (Advanced Accelerator Applications). Ghassan K. Abou-Alfa reports research support form Agenus, Arcus, Astra Zeneca, BioNtech, BMS, Elicio, Genentech/Roche, Helsinn, Parker Institute, Pertzye, Puma, QED, Yiviva and Joined and consulting support form Astellas, Astra Zeneca, Autem, Berry Genomics, BioNtech, Boehringer Ingelheim, BMS, Eisai, Exelixis, Fibriogen, Genentech/Roche, Helio, Incyte, Ipsen, Merck, Merus, Neogene, Newbridge, Novartis, QED, Servier, Tempus, Thetis, Vector, Yiviva. Alice C. Wei reports research support from IPSEN and personal fees from Histosonics, AstraZeneca, Medtronic. Dr Drebin’s spouse is an employee of American Regent Pharmaceuticals. Dr Kemeny reported receiving funding from Amgen for research protocols not related to this study. Dr Cercek reported receiving research funding from AbbVie, Seattle Genetics, and serves as a paid member of the advisory boards of Bayer and Proteus.
Figures
References
-
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7-33. - PubMed
-
- Primrose JN, Fox RP, Palmer DH, et al. ; BILCAP study group. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20(5):663-673. - PubMed
-
- Valle J, Wasan H, Palmer DH, et al. ; ABC-02 Trial Investigators., Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273-1281. - PubMed
-
- Oh D-Y, Ruth He A, Qin S, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022;1(8):EVIDoa2200015. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

