Design of multivalent-epitope vaccine models directed toward the world's population against HIV-Gag polyprotein: Reverse vaccinology and immunoinformatics
- PMID: 39331650
- PMCID: PMC11432917
- DOI: 10.1371/journal.pone.0306559
Design of multivalent-epitope vaccine models directed toward the world's population against HIV-Gag polyprotein: Reverse vaccinology and immunoinformatics
Abstract
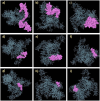
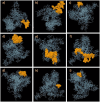
Significant progress has been made in HIV-1 research; however, researchers have not yet achieved the objective of eradicating HIV-1 infection. Accordingly, in this study, eucaryotic and procaryotic in silico vaccines were developed for HIV-Gag polyproteins from 100 major HIV subtypes and CRFs using immunoinformatic techniques to simulate immune responses in mice and humans. The epitopes located in the conserved domains of the Gag polyprotein were evaluated for allergenicity, antigenicity, immunogenicity, toxicity, homology, topology, and IFN-γ induction. Adjuvants, linkers, CTLs, HTLs, and BCL epitopes were incorporated into the vaccine models. Strong binding affinities were detected between HLA/MHC alleles, TLR-2, TLR-3, TLR-4, TLR-7, and TLR-9, and vaccine models. Immunological simulation showed that innate and adaptive immune cells elicited active and consistent responses. The human vaccine model was matched with approximately 93.91% of the human population. The strong binding of the vaccine to MHC/HLA and TLR molecules was confirmed through molecular dynamic stimulation. Codon optimization ensured the successful translation of the designed constructs into human cells and E. coli hosts. We believe that the HIV-1 Gag vaccine formulated in our research can reduce the challenges faced in developing an HIV-1 vaccine. Nevertheless, experimental verification is necessary to confirm the effectiveness of these vaccines in these models.
Copyright: © 2024 Hashempour et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


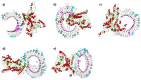


References
-
- Dehghani B, Hasanshahi Z, Hashempour T, Kazerooni PA (2021) Subtype classification by polymerase and gag genes of HIV-1 Iranian sequences registered in the NCBI GenBank. Current Proteomics 18: 153–161.
-
- Halaji M, Hashempour T, Moayedi J, Pouladfar GR, Khansarinejad B, et al. (2019) Viral etiology of acute respiratory infections in children in Southern Iran. Revista da Sociedade Brasileira de Medicina Tropical 52. - PubMed
-
- Hashempoor T, Alborzi AM, Moayedi J, Ajorloo M, Bamdad T, et al. (2018) A decline in anti-core+ 1 antibody titer occurs in successful treatment of patients infected with hepatitis C virus. Jundishapur Journal of Microbiology 11.
-
- Fani M, Sadooni R, Nejad MH, Mirzavieh NN, Mobarak S, et al. (2023) Two Oncoviruses of HPV and EBV in Breast Cancer: An Iran-based Study. Journal of Liaquat University of Medical & Health Sciences 22: 169–173.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

