Combined assembloid modeling and 3D whole-organ mapping captures the microanatomy and function of the human fallopian tube
- PMID: 39331707
- PMCID: PMC11430475
- DOI: 10.1126/sciadv.adp6285
Combined assembloid modeling and 3D whole-organ mapping captures the microanatomy and function of the human fallopian tube
Abstract
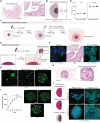
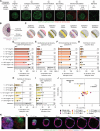
The fallopian tubes play key roles in processes from pregnancy to ovarian cancer where three-dimensional (3D) cellular and extracellular interactions are important to their pathophysiology. Here, we develop a 3D multicompartment assembloid model of the fallopian tube that molecularly, functionally, and architecturally resembles the organ. Global label-free proteomics, innovative assays capturing physiological functions of the fallopian tube (i.e., oocyte transport), and whole-organ single-cell resolution mapping are used to validate these assembloids through a multifaceted platform with direct comparisons to fallopian tube tissue. These techniques converge at a unique combination of assembloid parameters with the highest similarity to the reference fallopian tube. This work establishes (i) an optimized model of the human fallopian tubes for in vitro studies of their pathophysiology and (ii) an iterative platform for customized 3D in vitro models of human organs that are molecularly, functionally, and microanatomically accurate by combining tunable assembloid and tissue mapping methods.
Figures




References
-
- Croxatto H. B., Physiology of gamete and embryo transport through the fallopian tube. Reprod. Biomed. Online 4, 160–169 (2002). - PubMed
-
- Leese H., Tay J., Reischl J., Downing S., Formation of fallopian tubal fluid: Role of a neglected epithelium. Reproduction, 339–346 (2001). - PubMed
-
- Zondervan K. T., Becker C. M., Missmer S. A., Endometriosis. N. Engl. J. Med. 382, 1244–1256 (2020). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

