Estrogen signaling suppresses tumor-associated tissue eosinophilia to promote breast tumor growth
- PMID: 39331714
- PMCID: PMC11430468
- DOI: 10.1126/sciadv.adp2442
Estrogen signaling suppresses tumor-associated tissue eosinophilia to promote breast tumor growth
Abstract
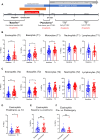
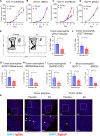
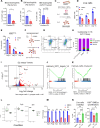
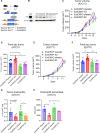
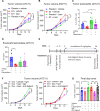
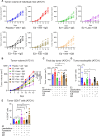
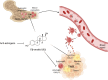
Estrogens regulate eosinophilia in asthma and other inflammatory diseases. Further, peripheral eosinophilia and tumor-associated tissue eosinophilia (TATE) predicts a better response to immune checkpoint blockade (ICB) in breast cancer. However, how and if estrogens affect eosinophil biology in tumors and how this influences ICB efficacy has not been determined. Here, we report that estrogens decrease the number of peripheral eosinophils and TATE, and this contributes to increased tumor growth in validated murine models of breast cancer and melanoma. Moreover, estrogen signaling in healthy female mice also suppressed peripheral eosinophil prevalence by decreasing the proliferation and survival of maturing eosinophils. Inhibiting estrogen receptor (ER) signaling decreased tumor growth in an eosinophil-dependent manner. Further, the efficacy of ICBs was increased when administered in combination with anti-estrogens. These findings highlight the importance of ER signaling as a regulator of eosinophil biology and TATE and highlight the potential near-term clinical application of ER modulators to increase ICB efficacy in multiple tumor types.
Figures

References
-
- Giaquinto A. N., Sung H., Miller K. D., Kramer J. L., Newman L. A., Minihan A., Jemal A., Siegel R. L., Breast cancer statistics, 2022. CA Cancer J. Clin. 72, 524–541 (2022). - PubMed
-
- Harbeck N., Penault-Llorca F., Cortes J., Gnant M., Houssami N., Poortmans P., Ruddy K., Tsang J., Cardoso F., Breast cancer. Nat. Rev. Dis. Primers. 5, 66 (2019). - PubMed
-
- Harbeck N., Bartlett M., Spurden D., Hooper B., Zhan L., Rosta E., Cameron C., Mitra D., Zhou A., CDK4/6 inhibitors in HR+/HER2- advanced/metastatic breast cancer: A systematic literature review of real-world evidence studies. Future Oncol. 17, 2107–2122 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

