Primaquine for uncomplicated Plasmodium vivax malaria in children younger than 15 years: a systematic review and individual patient data meta-analysis
- PMID: 39332427
- PMCID: PMC11480364
- DOI: 10.1016/S2352-4642(24)00210-4
Primaquine for uncomplicated Plasmodium vivax malaria in children younger than 15 years: a systematic review and individual patient data meta-analysis
Abstract
Background: Primaquine, the only widely available treatment to prevent relapsing Plasmodium vivax malaria, is produced as 15 mg tablets, and new paediatric formulations are being developed. To inform the optimal primaquine dosing regimen for children, we aimed to determine the efficacy and safety of different primaquine dose strategies in children younger than 15 years.
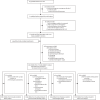
Methods: We undertook a systematic review (Jan 1, 2000-July 26, 2024) for P vivax efficacy studies with at least one treatment group that was administered primaquine over multiple days, that enrolled children younger than 15 years, that followed up patients for at least 28 days, and that had data available for inclusion by June 30, 2022. Patients were excluded if they were aged 15 years or older, presented with severe malaria, received adjunctive antimalarials within 14 days of diagnosis, commenced primaquine more than 7 days after starting schizontocidal treatment, had a protocol violation in the original study, or were missing data on age, sex, or primaquine dose. Available individual patient data were collated and standardised. To evaluate efficacy, the risk of recurrent P vivax parasitaemia between days 7 and 180 was assessed by time-to-event analysis for different total mg/kg primaquine doses (low total dose of ∼3·5 mg/kg and high total dose of ∼7 mg/kg). To evaluate tolerability and safety, the following were assessed by daily mg/kg primaquine dose (low daily dose of ∼0·25 mg/kg, intermediate daily dose of ∼0·5 mg/kg, and high daily dose of ∼1 mg/kg): gastrointestinal symptoms (vomiting, anorexia, or diarrhoea) on days 5-7, haemoglobin decrease of at least 25% to less than 7g/dL (severe haemolysis), absolute change in haemoglobin from day 0 to days 2-3 or days 5-7, and any serious adverse events within 28 days. This study is registered with PROSPERO, CRD42021278085.
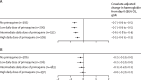
Findings: In total, 3514 children from 27 studies and 15 countries were included. The cumulative incidence of recurrence by day 180 was 51·4% (95% CI 47·0-55·9) following treatment without primaquine, 16·0% (12·4-20·3) following a low total dose of primaquine, and 10·2% (8·4-12·3) following a high total dose of primaquine. The hazard of recurrent P vivax parasitaemia in children younger than 15 years was reduced following primaquine at low total doses (adjusted hazard ratio [HR] 0·17, 95% CI 0·11-0·25) and high total doses (0·09, 0·07-0·12), compared with no primaquine. In 525 children younger than 5 years, the relative rates of recurrence were also reduced, with an adjusted HR of 0·33 (95% CI 0·18-0·59) for a low total dose and 0·13 (0·08-0·21) for a high total dose of primaquine compared with no primaquine. The rate of recurrence following a high total dose was reduced compared with a low dose in children younger than 15 years (adjusted HR 0·54, 95% CI 0·35-0·85) and children younger than 5 years (0·41, 0·21-0·78). Compared with no primaquine, children treated with any dose of primaquine had a greater risk of gastrointestinal symptoms on days 5-7 after adjustment for confounders, with adjusted risks of 3·9% (95% CI 0-8·6) in children not treated with primaquine, 9·2% (0-18·7) with a low daily dose of primaquine, 6·8% (1·7-12·0) with an intermediate daily dose of primaquine, and 9·6% (4·8-14·3) with a high daily dose of primaquine. In children with 30% or higher glucose-6-phosphate dehydrogenase (G6PD) activity, there were few episodes of severe haemolysis following no primaquine (0·4%, 95% CI 0·1-1·5), a low daily dose (0·0%, 0·0-1·6), an intermediate daily dose (0·5%, 0·1-1·4), or a high daily dose (0·7%, 0·2-1·9). Of 15 possibly drug-related serious adverse events in children, two occurred following a low, four following an intermediate, and nine following a high daily dose of primaquine.
Interpretation: A high total dose of primaquine was highly efficacious in reducing recurrent P vivax parasitaemia in children compared with a low dose, particularly in children younger than 5 years. In children treated with high and intermediate daily primaquine doses compared with low daily doses, there was no increase in gastrointestinal symptoms or haemolysis (in children with 30% or higher G6PD activity), but there were more serious adverse events.
Funding: Medicines for Malaria Venture, Bill & Melinda Gates Foundation, and Australian National Health and Medical Research Council.
Copyright © 2024 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests JKB reports institutional research funding from MMV, GSK, Wellcome Trust, and Sanaria; participation on the US National Institutes of Health data safety monitoring board; and membership of the editorial board of Travel Medicine and Infectious Disease and the guidelines development group for malaria control and elimination, Global Malaria Programme, WHO. RJC, JKB, and RNP report contributions to Up-to-Date. All other authors declare no competing interests.
Figures


Comment in
-
Primaquine for children, once and for all.Lancet Child Adolesc Health. 2024 Nov;8(11):775-777. doi: 10.1016/S2352-4642(24)00231-1. Epub 2024 Sep 24. Lancet Child Adolesc Health. 2024. PMID: 39332424 No abstract available.
References
-
- WHO 22nd Invitation to Manufacturers of Antimalarial Medicine to Submit an Expression of Interest (EOI) for Product Evaluation to the WHO Prequalification Unit (PQT). Geneva, Switzerland, 2023. https://extranet.who.int/prequal/sites/default/files/document_files/EOI-...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

